Abstract
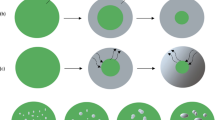
The thermochemical approach to analysis of thermal decomposition of solids, developed earlier by L’vov, is extended here, for the first time, to interpret the kinetics and mechanism of the reduction of an oxide (NiO) by a gas (H2). This approach is based on the mechanism of congruent dissociative vaporization of the reactant, Langmuir kinetics and determination of the Arrhenius E parameter by the third-law method. The calculated enthalpy of the reaction is in good agreement with the experimentally measured E value. Many other mechanistic and kinetic features of the reaction are explained within the framework of the given theoretical approach. These include: the formation of metal nuclei; the initial autocatalytic behavior; the formation of nanocrystalline structure of the reduced metal product; the equimolar and isobaric modes of reduction; the dependence of reduction rate on hydrogen pressure; the more than twofold decrease of the E parameter with the extent of reaction α, and the systematic increase of E with temperature.
Similar content being viewed by others
References
Benton AF, Emmett PH. The reduction of nickelous and ferric oxides by hydrogen. J Am Chem Soc. 1924;46:2728–37.
Chufarov GI, Zhuravleva MG, Tatievskaya EP. Reduction and dissociation of cobalt and nickel oxides. Dokl Akad Nauk SSSR. 1950;73:1209–12 (in Russian).
Kivnick A, Hixson AN. The reduction of nickel oxide in fluidized-bed. Chem Eng Prog. 1952;48:394–400.
Parravano G. The reduction of nickel oxide by hydrogen. J Am Chem Soc. 1952;74:1194–8.
Hauffe K, Rahmel A. Zur Kinetik der Reduktion von Nickeloxyd mit Wasserstoff. Z Phys Chem. 1954;1:104–28.
Delmon B. La cinétique des réactions hétérogènes. Recherche des méthods pour l’étude de la réduction de l’oxyde de nickel par l’hydrogène. Bull Soc Chim Fr. 1961;590–7.
Bandrowski J, Bickling CR, Yang KH, Hougen OA. Kinetics of the reduction of nickel oxide by hydrogen. Chem Eng Sci. 1962;17:379–90.
Yamaguchi A, Moriyama J. Reduction kinetics of NiO powder by hydrogen. J Jpn Inst Met. 1964;28:692–7.
Rozhdestvenskii VP, Volgina LM, Strokova TP. About interaction of hydrogen with nickel oxide. Zh Prikl Khim. 1967;40:705–11 (in Russian).
Frety D. Ph.D. Thesis. Université de Lyon; 1969.
Delmon B. Introduction a la Cinétique Hétérogène. Paris: Technip; 1969.
Chiesa F, Rigaud M. La reduction de l’oxyde de nickel par l’hydrogène. Can J Chem Eng. 1971;49:617–20.
Kurosawa T, Hasegawa R, Yagihashi T. Hydrogen reduction of nickel oxide under high pressure. Trans Jpn Inst Met. 1972;13:265–71.
Szekely CI, Lin HY, Soh A. Structural model for gas-solid reactions with a moving boundary-V. An experimental study of the reduction of porous nickel-oxide pellets with hydrogen. Chem Eng Sci. 1973;28:1975–89.
Evans JW, Haase K. Measurement of the rate of reduction reactions by the torsion technique. High Temp Sci. 1976;8:167–77.
Nakajima M, Shimiza S, Onuki K, Ikezoe Y, Sato S. Chem Soc Jpn. 1989;62:681 (cited in [18]).
Rashed AH, Rao YK. Kinetics of reduction of nickel oxide with hydrogen gas in the 230–452°C range. Chem Eng Commun. 1996;156:1–30.
Richardson JT, Scates R, Twigg MV. X-ray study of nickel oxide reduction by hydrogen. Appl Catal A. 2003;246:137–50.
Richardson JT, Scates R, Twigg MV. X-ray diffraction study of the hydrogen reduction of NiO/α-Al2O3 steam reforming catalysts. Appl Catal A. 2004;267:35–46.
Utigard TA, Wu M, Plascencia G, Marin T. Reduction kinetics of Goro nickel oxide using hydrogen. Chem Eng Sci. 2005;60:2061–8.
Janković B, Adnadević B, Mentus S. The kinetic analysis of non-isothermal nickel oxide reduction in hydrogen atmosphere using the invariant kinetic parameters method. Thermochim Acta. 2007;456:48–55.
Janković B, Adnadević B, Mentus S. The kinetic study of temperature-programmed reduction of nickel oxide in hydrogen atmosphere. Chem Eng Sci. 2008;63:567–75.
Erri P, Varma A. Diffusional effects in nickel oxide reduction kinetics. Ind Eng Chem Res. 2009;48:4–6.
L’vov BV. The mechanism of the thermal decomposition of metal nitrates in graphite furnaces for atomic absorption analysis. Zh Anal Khim. 1990;45:2144–53 (in Russian).
L’vov BV. Mechanism of the thermal decomposition of metal nitrates from graphite furnace mass spectrometry studies. Mikrochim Acta (Wien) II. 1991;299–308.
L’vov BV. Book of abstracts, Invited lecture at XXVII CSI, Bergen; 1991. p. A-5.2.
L’vov BV. Thermal decomposition of solid and liquid substances. St. Petersburg: Politekh. Univ; 2006 (in Russian).
L’vov BV. Thermal decomposition of solids and melts: new thermochemical approach to the mechanism, kinetics and methodology. Berlin: Springer; 2007.
L’vov BV. Kinetics and mechanism of solid decompositions—from basic discoveries by atomic absorption spectrometry and quadrupole mass spectroscopy to thorough thermogravimetric analysis. Spectrochim Acta B. 2008;63:332–6.
L’vov BV. Fundamental restrictions of the second-law and Arrhenius plot methods used in the determination of reaction enthalpies in decomposition kinetics. J Therm Anal Calorim. 2008;92:639–42.
L’vov BV. Role of vapour oversaturation in the thermal decomposition of solids. J Therm Anal Calorim. 2009;96:321–30.
L’vov BV. Thermochemical approach to solid-state decomposition reactions against the background of traditional theories. J Therm Anal Calorim. 2009;96:487–93.
L’vov BV. Impact of the gaseous environment on the kinetics of solid-state decompositions. J Therm Anal Calorim. 2010;100:967–75.
Volmer M. Über Keimbildung und Keimwirkung als Spezialfälle der heterogenen Katalyse. Z Elektrochem. 1929;35:555–61.
L’vov BV. The mechanism of solid-state decompositions in a retrospective. J Therm Anal Calorim. 2010;101:1175–82.
Kireev VA. Practical calculation in chemical thermodynamics. Moscow: Khimiya; 1975 (in Russian).
Ryabin VA, Ostroumov VA, Svit TF. Thermodynamic constants of substances. Reference book. Leningrad: Khimiya; 1977 (in Russian).
Gurvich LV, Veits IV, Medvedev VA, et al. Thermodynamic constants of individual substances. Reference book, vol 4. Moscow: Nauka; 1979 (in Russian).
Janković B. Isothermal reduction of NiO using hydrogen: conventional and Weibull kinetic analysis. J Phys Chem Solids. 2007;68:2233–46.
L’vov BV. Interpretation of atomization mechanisms in electrothermal atomic absorption spectrometry by analysis of the absolute rates of the processes. Spectrochim Acta B. 1997;52:1–23.
L’vov BV, Fernandes GHA. Regularities in thermal dissociation of oxides in graphite furnaces for atomic absorption analysis. Zh Anal Khim. 1984;39:221–31 (in Russian).
Nikolsky BP, editor. Handbook of chemist, vol 1. Leningrad: Khimia; 1961 (in Russian).
L’vov BV, Nikolaev VG. Calculation of diffusion coefficients of metal vapors in argon as applied to the problems of ET AAS. Zh Prikl Spectosk. 1987;46:7–12 (in Russian).
L’vov BV. Application of the third-law methodology to investigation of decomposition kinetics. Thermochim Acta. 2004;424:183–9.
L’vov BV. How to improve efficiency of thermal analysis in decomposition kinetics. J Therm Anal Calorim. 2005;79:151–6.
L’vov BV. Mechanism of thermal decomposition of alkaline-earth carbonates. Thermochim Acta. 1997;303:161–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
L’vov, B.V., Galwey, A.K. The mechanism and kinetics of NiO reduction by hydrogen. J Therm Anal Calorim 110, 601–610 (2012). https://doi.org/10.1007/s10973-011-2000-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2000-0