Abstract
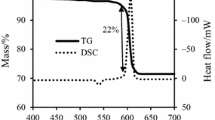
Thermal behavior of KClO4/Mg pyrotechnic mixtures heated in air was investigated by thermal analysis. Effects of oxygen balance and heating rates on the TG–DSC curves of mixtures were examined. Results showed that DSC curves of the mixtures had two exothermic processes when heated from room temperature to 700 °C, and TG curve exhibited a slight mass gain followed by a two-stage mass fall and then a significant mass increase. The exothermic peak at lower temperature and higher temperature corresponded to the ignition process and afterburning process, respectively. Under the heating rate of 10 °C min−1, the peak temperatures for ignition and afterburning process of stoichiometric KClO4/Mg (58.8/41.2) was 543 and 615 °C, respectively. When Mg content increased to 50%, the peak ignition temperature decreased to 530 °C, but the second exothermic peak changed little. Reaction kinetics of the two exothermic processes for the stoichiometric mixture was calculated using Kissinger method. Apparent activation energies for ignition and afterburning process were 153.6 and 289.5 kJ mol−1, respectively. A five-step reaction pathway was proposed for the ignition process in air, and activation energies for each step were also calculated. These results should provide reference for formula design and safety storage of KClO4/Mg-containing pyrotechnics.







Similar content being viewed by others
References
Conkling JA. Chemistry of pyrotechnics: basic principles and theory. New York: Marcel Dekker Inc.; 1985.
Hosseini SG, Eslami A. Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems. J Therm Anal Calorim. 2010;101:1111–9.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;141:322–6.
Lee JS, Hsu CK. The DSC studies on the phase transition, decomposition and melting of potassium perchlorate with additives. Thermochim Acta. 2001;367–368:367–70.
Barišin D, Haberle IB. Aging of pyrotechnic compositions. The investigation of chemical changes by IR spectroscopy and x-ray diffraction. Propellant Explos Pyrotech. 1989;14:162–9.
Tuukkanen IM, Charsley EL, Laye PG, Rooney JJ, Griffiths TT, Lemmetyinen H. Pyrotechnic and thermal studies on the magnesium-strontium nitrate pyrotechnic system. Propellant Explos Pyrotech. 2006;31:110–5.
Tuukkanen IM, Brown SD, Charsle EL, Goodall SJ, Rooney JJ, Griffith TT, Lemmetyinen H. Studies on the ageing of a magnesium-strontium nitrate pyrotechnic composition using isothermal microcalorimetry and thermal analysis techniques. Thermochim Acta. 2004;417:223–9.
Knowlton GD, Ludwig CP. Low temperature autoignition composition. US Patent, 6479702, 2004.
Krone U. Pyrotechnical mixture for producing a smoke screen. US Patent, 4968365, 1990.
Salzano E, Basco A, Cammarota F. Confined after-burning of display pyrotechnics and explosives. In: 32nd Combustion meeting, Napoli (IT), 2009.
Donhaue L, Pegg MJ, Zhang F. Afterburning of TNT detonation products in air. In: Seventh international symposium on hazards, prevention, and mitigation of industrial explosives (7th ISGHPMIE). St. Petersburg, Russia, 2008.
Basco A, Salzano E. The risk of storage plant of pyrotechnics. Chem Eng Trans. 2010;19:231–6.
Pourmortazavi SM, Fathollahi M, Hajimirsadeghi SS, Hosseini SG. Thermal behavior of aluminum powder and potassium perchlorate mixtures by DTA and TG. Thermochim Acta. 2006;443:129–31.
Sharma TP, Varshney VS, Kumar S. Products of combustion of the metal powders. Fire Sci Technol. 1992;12:29–38.
Ravindran N, Chattopadhyay DK, Zakula A, Battocchi D, Webster DC, Bierwagen GP. Thermal stability of magnesium-rich primers based on glycidyl carbamate resins. Polym Degrad Stab. 2010;95:1160–6.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244–51.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Barral L, Cano J, Lopez J, Lopez-Bueno I, Nogueira P, Ramirez C, et al. Thermogravimetric study of tetrafunctional/phenol novolac epoxy mixtures cured with a diamine. J Therm Anal Calorim. 1998;51:489–501.
Chrissafis K. Kinetics of thermal degradation of polymers-Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Gao M, Wu W, Yan Y. Thermal degradation and flame retardancy of epoxy resins containing intumescent flame retardant. J Therm Anal Calorim. 2009;95:605–8.
Tiptipakorn S, Damrongsakkul S, Ando S, Hemvichian K, Rimdusit S. Thermal degradation behaviors of polybenzoxazine and silicon-containing polyimide blends. Polym Degrad Stab. 2007;92:1265–78.
Boey FYC, Qiang W. Experimental modeling of the cure kinetics of an epoxy-hexaanhydro-4-methylphthalicanhydride (MHHPA) system. Polymer. 2000;41:2081–94.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Appl Polym Sci B. 1966;4–5:323–8.
Shih TS, Wang JH, Chong KZ. Combustion of magnesium alloys in air. Mater Chem Phys. 2004;2–3:302–9.
Tribelhorn MJ, Venables DS, Brown ME. Combustion of some zinc-fuelled binary pyrotechnic systems. Thermochim Acta. 1995;256:309–24.
Lee JS, Hsu CK. The effect of different zirconium on thermal behaviors for Zr/KClO4 priming composition. Thermochim Acta. 2001;367–368:375–9.
Solymosi F. Structure and stability of salts of halogen oxyacids in the solid phase. New York: Wiley; 1977.
Mclain JH. Pyrotechnics from the viewpoint of solid state chemistry. Philadelphia: Franklin Institute; 1980, p. 20.
Acknowledgements
The authors would like to acknowledge the support of National High Technology Development Program of China under Grant No. 2009AA8043022.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, X., Zhang, J., Zhang, Q. et al. Studies on ignition and afterburning processes of KClO4/Mg pyrotechnics heated in air. J Therm Anal Calorim 109, 1333–1340 (2012). https://doi.org/10.1007/s10973-011-1991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1991-x