Abstract
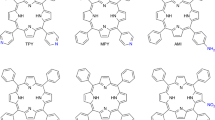
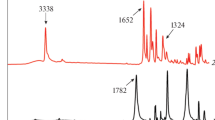
Crystalline structure, thermo-oxidative and thermal stability of symmetrical and asymmetrical piperidyl and morpholinyl derivatives of both N-substituted and non-N-substituted butyl diphenyl-diketo-pyrrolopyrrole (DPP) pigments were studied using differential scanning calorimetry (DSC) and thermogravimetry (TG). Except for the asymmetrical morpholine DPP derivative, all the samples showed melting peaks which were relatively close to their degradation temperatures (from 260 to 430 °C). Using DSC, monotropic polymorphism was revealed in the symmetrical piperidyl-N-butyl-derivative which confirmed earlier observation about tendency of symmetrical N-alkyl DPP derivates to form several crystalline structures. TG carried out under nitrogen atmosphere served for distinguishing of evaporation/sublimation and degradation temperatures. Temperatures of evaporation/sublimation were typically 10–30 °C lower in comparison with temperatures of thermal degradation. The highest thermal (450 °C) and thermo-oxidative stability (around 360 °C) showed the DPP derivatives containing morpholine moieties with no alkyl substitution on NH-group of DPP core. The presence of the latter was found to be the most destabilizing factor. Piperidyl group showed more stabilizing effect due to its polar character and its influence on π–π intermolecular interactions of neighbouring phenyl groups. The highest stabilizing effect of morpholine moiety on DPP structure was explained based on the presence of polar oxygen atom in that group. The preparations of 3,6-di-(4-morpholinophenyl)-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione and 3-(phenyl)-6-(4-morpholinophenyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione are reported.





Similar content being viewed by others
References
Vala M, Weiter M, Vyňuchal J, Toman P, Luňák S Jr. Comparative studies of diphenyl-diketo-pyrrolopyrrole derivatives for electroluminescence applications. J Fluoresc. 2008;18:1181–5.
Mizuguchi J. Correlation between crystal and electronic structures in diketopyrrolopyrrole pigments as viewed from exciton coupling effects. J Phys Chem A. 2000;104:1817–21.
Hoki T, Takahashi H, Suzuki S, Mizuguchi J. Hydrogen gas sensor based upon proton acceptors integrated in copper-tetra-2, 3-pyridinoporphyradine. IEEE Sensors J. 2007;7:808–13.
Beyerlein T, Tieke B, Forero-Lenger S, Brütting W. Red electroluminescence from a 1, 4-diketopyrrolo[3, 4-c]pyrrole (DPP)-based conjugated polymer. Synthetic Metals. 2002;130:115–9.
Potrawa T, Langhals H. Fluorescent dyes with large Stokes shifts - soluble dihydropyrrolopyrrolediones. Chemische Berichte. 1987;120:1075–8.
Fukuda M, Kodama K, Yamamoto H, Mito K. Evaluation of new organic pigments as laser-active media for a solid-state dye laser. Dyes Pigments. 2004;63:115–25.
Luňák S, Vyňuchal J, Vala M, Havel L, Hrdina R. The synthesis, absorption and fluorescence of polar diketo-pyrrolo-pyrroles. Dyes Pigments. 2009;82:102–8.
David J, Weiter M, Vala M, Vyňuchal J, Kučerík J. Stability and structural aspects of diketopyrrolopyrrole pigment and its N-alkyl derivatives. Dyes Pigments. 2011;89:137–43.
Vala M, Vyňuchal J, Toman P, Weiter M, Luňák S Jr. Novel, soluble diphenyl-diketo-pyrrolopyrroles: Experimental and theoretical study. Dyes Pigments. 2010;84:176–82.
Weiter M, Salyk O, Bednář P, Vala M, Navrátil J, Zmeškal O, Vyňuchal J, Luňák S Jr. Morphology and properties of thin films of diketopyrrolopyrrole derivatives. Mat Sci Eng B. 2009;165:148–52.
Qiao Z, Xu Y, Lin S, Peng J, Cao D. Synthesis ands characterization of red-emitting diketopyrrolopyrrole-alt-phenylenevinylene polymers. Synthetic Metals. 2010;160:1544–50.
Palai AK, Mishra SP, Kumar A, Srivastava R, Kamalasana MP, Patri M. Synthesis and characterization of alternative donor-acceptor arranged poly(arylene enthylene)s derived from 1, 4-diketo-3, 6-diphenylpyrrolo[3, 4-c]pyrrole (DPP). Eur Polym J. 2010;46:1940–51.
Mizuguchi J, Imoda T, Takahashi H, Yamakami H. Polymorph of 1, 4-diketo-3, 6-bis-(4′-dipyridyl)-pyrrolo-[3, 4-c]pyrrole and their hydrogen bond network: A material for H2 gas sensor. Dyes Pigments. 2006;68:47–52.
Rotaru A, Moanta A, Popa G, Rotaru P, Segal. E. Thermal decomposition kinetics of some aromatic azomonoethers. full access. Part IV. Non-isothermal kinetics of 2-allyl-4-((4-(4-methylbenzyloxy)phenyl)diazenyl)phenol in air flow. J Therm Anal Calorim. 2009;97:485–91.
Rotaru A, Moanta A, Rotaru P, Segal E. Thermal decomposition kinetics of some aromatic azomonoethers Part III. Non-isothermal study of 4-[(4-chlorobenzyl)oxy]-4′-chloroazobenzene in dynamic air atmosphere. J Therm Anal Calorim. 2009;95:161–6.
Acknowledgements
The financial support of the Ministry of Education of the Czech Republic - project MSM 0021630501, Academy of Sciences of the Czech Republic project KAN401770651 and Czech Science Foundation project GACR 203/08/1594 are acknowledged. This study was also supported by the project “Centre for Materials Research at FCH BUT” No. CZ.1.05/2.1.00/01.0012 from ERDF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kučerík, J., David, J., Weiter, M. et al. Stability and physical structure tests of piperidyl and morpholinyl derivatives of diphenyl-diketo-pyrrolopyrroles (DPP). J Therm Anal Calorim 108, 467–473 (2012). https://doi.org/10.1007/s10973-011-1896-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1896-8