Abstract
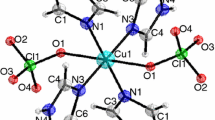
Bis(1-octylammonium) tetrachlorocuprate (1-C8H17NH3)2CuCl4(s) was synthesized by the method of liquid phase reaction. The crystal structure of the compound has been determined by X-ray crystallography. The lattice potential energy was obtained from the crystallographic data. Molar enthalpies of dissolution of (1-C8H17NH3)2CuCl4(s) at various molalities were measured at 298.15 K in the double-distilled water by means of an isoperibol solution-reaction calorimeter, respectively. In terms of Pitzer’s electrolyte solution theory, the molar enthalpy of dissolution of (1-C8H17NH3)2CuCl4(s) at infinite dilution was determined to be \( \Updelta_{\rm s} H_{\text{m}}^{\infty } = \, - 5. 9 7 2\,{\text{kJ}}\,{\text{mol}}^{ - 1} , \) and the sums of Pitzer’s parameters \( (4\beta_{{{\text{C}}_{ 8} {\text{H}}_{ 1 7} {\text{NH}}_{ 3} , {\text{Cl}}}}^{ ( 0 )L} + 2\beta_{\text{Cu,Cl}}^{ ( 0 )L} + \theta_{{{\text{C}}_{ 8} {\text{H}}_{ 1 7} {\text{NH}}_{ 3} , {\text{Cu}}}}^{L} ) \) and \( (2\beta_{{{\text{C}}_{ 8} {\text{H}}_{ 1 7} {\text{NH}}_{ 3} , {\text{Cl}}}}^{ ( 1 )L} + \beta_{\text{Cu,Cl}}^{ ( 1 )L} ) \) were obtained.




Similar content being viewed by others
References
Jakubas R, Bator G, Gosniowska M, Ciunik Z, Baran J, Lefebvre J. Crystal structure and phase transition of [(CH3)2NH2]GaCl4. J Phys Chem Solids. 1997;58:989–98.
Li WP, Zhang DS, Zhang TP, Wang TZ, Ruan DS, Xing DQ, Li HB. Study of solid–solid phase change of (n-C n H2n+1NH3)2MCl4 for thermal energy storage. Thermochim Acta. 1999;326:183–6.
Wu KZ, Zhang JJ, Liu XD. Subsolidus phase diagram of binary system of thermotropic phase transitions compounds (n-C n H2n+1NH3)2MnCl4 (n = 12, 14, 16). Thermochim Acta. 2009;483:55–7.
Kang JK, Choy JH, Madeleine RL. Phase transition behavior in the perovskite-type layer compound (n-C12H25NH3)2CuCl4. J Phys Chem Solids. 1993;54:1567–77.
Yang WW, Di YY, Li J, Kong YX. Thermochemistry on ephedrine hydrochloride and N-methylephedrine hydrochloride. J Chem Thermodyn. 2009;41:945–50.
Dan WY, Di YY, Kong YX, Wang Q, Yang WW, Wang DQ. Crystal structure and solid–solid phase transition of the complex (C11H18NO)2CuCl4(s). J Therm Anal Calorim. 2010;102:291–6.
Xue BD, Yang Q, Chen SP, Gao SL. Synthesis, crystal structure, and thermodynamics of a high-nitrogen copper complex with N,N-bis-(1(2)H-tetrazol-5-yl) amine. J Therm Anal Calorim. 2010;101:997–1002.
Di YY, Tan ZC, Li LW, Gao SL. Thermochemistry on the coordination compounds of zinc sulphate with several L-a-amino acids. J Chem Thermodyn. 2006;38:884–8.
Liu YP, Di YY, Dan WY, He DH, Kong YX, Yang WW. Thermochemistry on dodecylamine hydrochloride and bis-dodecylammonium tetrachlorozincate. J Therm Anal Calorim. 2011;103:987–93.
Jenkins HDB, Glasser L. Ionic Hydrates, M p X q ·nH2O: lattice energy and standard enthalpy of formation estimation. Inorg Chem. 2002;41:378–88.
Kong YX, Di YY, Yang WW, Lü YF, Tan ZC. Crystal structure, phase transition, and thermodynamic properties of bis-dodecylammonium tetrachlorozincate (C12H25NH3)2ZnCl4(s). Chin J Chem. 2010;28:521–30.
Yang JZ, Pitzer KS. Thermodynamics of electrolyte mixtures. Activity and osmotic coefficients consistent with the higher-order limiting law for symmetrical mixing. J Solut Chem. 1988;17:909–24.
Phutela RC, Pitzer KS. Thermodynamics of electrolyte mixtures. Enthalpy and the effect of temperature on the activity coefficient. J Solut Chem. 1986;15:649–62.
Pitzer KS. Activity coefficients in electrolyte solutions. 2nd ed. Boca Raton: CRC Press; 1991. p. 75–153.
Pitzer KS. Thermodynamics of unsymmetrical electrolyte mixtures. Enthalpy and heat capacity. J Phys Chem. 1983;87:2360–4.
Yang WW, Di YY, Kong YX, Tan ZC. Low-temperature heat capacities and standard molar enthalpy of formation of pyridine-2,6-dicarboxylic acid. Chin Phys B. 2010;19:060517-1–7.
Acknowledgements
This study is financially supported by the National Natural Science Foundations of China under the contract NSFC No. 20673050 and 20973089.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y.P., Di, Y.Y., Dan, W.Y. et al. Crystal structure and thermochemical properties of bis(1-octylammonium) tetrachlorocuprate. J Therm Anal Calorim 109, 287–293 (2012). https://doi.org/10.1007/s10973-011-1737-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1737-9