Abstract
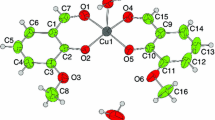
In this study, simultaneous TG/DTG-DTA technique was used for two cobalt(II) complexes with neocuproine(neoc) and the anion of a substituted salicylaldehyde ligand (X-salo) (X = 3-OCH3, or 5-CH3) with the general formula [Co(X-salo)2(neoc)], to determine their thermal degradation in inert atmosphere, which was found to be a multi-step decomposition related to the release of the ligand molecules. The solid material at 1300 °C (verified with PXRD) was a mixture of carbonaceous metal cobalt. Evolved gas analysis by coupled TG-MS verified the elimination of a formaldehyde molecule in the first decomposition stage, initially proposed by the percentage mass loss data. By single-crystal X-ray diffraction analysis an octahedral geometry of the complex [Co(3-OCH3-salo)2(neoc)] was found. The variable temperature magnetic susceptibility measurements showed a paramagnetic nature of the complexes, in accordance with their molecular structure. Finally, for the determination of the activation energy (E) two different methods (the isoconversional methods of Ozawa, Flynn and Wall (OFW) and Friedman) were used comparatively.










Similar content being viewed by others
References
Prasad RN, Agrawal A. Synthesis and spectroscopic studies of mixed ligand complexes of cobalt(II) with salicylaldehyde, hydroxyarylketones and beta-diketones. J Indian Chem Soc. 2006;83(1):75–7.
Hussain ST, Ahmad H, Atta MA, Afzal M, Saleem M. High performance liquid chromatography (HPLC), atomic absorption spectroscopy (AAS) and infrared spectroscopy determination and solvent extraction of uranium, using bis(salicylaldehyde) propylene diamine as complexing agent. J Trace Microprobe Tech. 1998;16(2):139–49.
Sajith P, Ummer MT, Mandal N, Mandot SK, Agrawal SL, Bandyopadhyay S, Mukhopadhyay R, D’Cruz B, Deuri AS, Kuriakose P. Synthesis of cobalt complexes and their evaluation as an adhesion promoter in a rubber-steel wire system. J Adhesion Sci Technol. 2005;19(16):1475–91.
Sun Y-X, Gao G-Z. Bis(4-bromo-2-formylphenolato-k 2 O,O′)copper(II). Acta Cryst. 2005;E61(2):m354–5.
Chen Q. Bis(4-bromo-2-formylphenolato-k 2 O,O′)zinc(II). Acta Cryst. 2006;E62(1):m56–7.
Yang Y-M, Lu P-C, Zhu T-T, Liu C-H. Bis(2-formylphenolato-k 2 O,O′)iron(II). Acta Cryst. 2007;E63(6):m1613.
Pessoa JC, Cavaco I, Correira I, Tomaz I, Duarte T, Matias PM. Oxovanadium(IV) complexes with aromatic aldehydes. J Inorg Biochem. 2000;80(1):35–9.
Lalia-Kantouri M, Papadopoulos CD, Hatzidimitriou AG, Skoulika S. Hetero-heptanuclear (Fe–Na) complexes of salicylaldehydes: crystal and molecular structure of [Fe2(3-OCH3-salo)8/Na5]·3OH·8H2O. Struct Chem. 2009;20(2):177–84.
Lalia-Kantouri M, Dimitriadis T, Papadopoulos CD, Gdaniec M, Czapik A, Hatzidimitriou AG. Synthesis and structural characterization of iron(III) complexes with 2-hydroxyphenones. Z Anorg Allg Chem. 2009;635(13):2185–90.
Bedell SA, Martell AE. Oxidation of 2,6-di-tert-butylphenol by molecular oxygen. Catalysis by tetrakis(bipyridyl)(μ-peroxo)(μ-hydroxo)dicobalt(III). Inorg Chem. 1983;22(2):364–7.
Papadopoulos CD, Hatzidimitriou AG, Voutsas GP, Lalia-Kantouri M. Synthesis and characterization of new addition compounds of bis(substituted-salicylaldehydo) cobalt(II) with 2,2′-bipyridine (bipy). Crystal and molecular structures of [CoII(3-methoxy-salicylaldehyde)2(bipy)]·CH3OH (1) and [CoII(bipy)3]Br2·0.5(5-chloro-salicylaldehydeH). 1.5CH3OH (5). Polyhedron. 2007;26(5):1077–86.
Papadopoulos CD, Lalia-Kantouri M, Jaud J, Hatzidimitriou AG. Substitution effect on new Co(II) addition compounds with salicylaldehydes and the nitrogenous bases phen or neoc: crystal and molecular structures of [CoII(5-NO2-salicylaldehyde)2(phen)], [CoII(5-CH3-salicylaldehyde)2(neoc)] and [CoII(5-Cl-salicylaldehyde)2(neoc)]. Inorg Chim Acta. 2007;360(11):3581–9.
Papadopoulos CD, Hatzidimitriou AG, Quirós M, Sigalas MP, Lalia-Kantouri M. Synthesis, characterization, thermal and theoretical studies of cobalt(II) addition compounds with 2-hydroxy-phenones and α-diimines. Crystal and molecular structures of [Co(2-hydroxy- benzophenone)2(bipy)]·2-hydroxy-benzophenoneH (3) and [Co(2-hydroxy- benzophenone)2(phen)] (8). Polyhedron, 2011; 30(3):486–96. doi:10.1016/j.poly.2010.11.010.
Curtis SA, Kurdziel K, Materazzi S, Vecchio S. Crystal structure and thermoanalytical study of a manganese(II) complex with 1-allylimidazole. J Therm Anal Calorim. 2008;92(1):109–14.
Dziewulska-Kulaczkowska A, Mazur L, Ferenc W. Thermal, spectroscopic and structural studies of zinc(II) complex with Nicotinamide. J Therm Anal Calorim. 2009;96(1):255–60.
Ye HM, Ren N, Li H, Zhang JJ, Sum SJ, Tian L. Synthesis, crystal structure and thermal decomposition kinetics of complex [Nd(BA)3bipy]2. J Therm Anal Calorim. 2010;101(1):205–11.
Figgis BN, Nyholm RS. A convenient solid for calibration of Gouy magnetic susceptibility apparatus. J Chem Soc. 1958;4190:1.
Oxford Diffraction program name(s), CrysAlis CCD and CrysAlis RED Ver.1.171.31. Abingdon, Oxfordshire, England: Oxford Diffraction Ltd.; 2006.
Sheldrick GM. SHELXS-97, program for solution of crystal structures, and SHELXL-97 program for crystal structures refinement. Göttingen: University of Göttingen; 1997.
Sheldrick GM. A short history of SHELX. Acta Cryst. 2008;A64:112–22.
O’Coonor JCh. Progress in inorganic chemistry. New York: Wiley; 1982. p. 204–83.
Martin RL. New pathways in inorganic chemistry. Cambridge: Cambridge University Press; 1968. p. 149–231.
Figgis NB, Lewis J. Progress in inorganic chemistry. New York: Interscience; 1964. p. 37.
Burger K. Coordination chemistry: experimental methods. Budapest: Akademiai Kiado; 1973.
Earnshaw A. Introduction to magnetochemistry. London: Academic Press; 1968.
Cotton FA, Wilkinson G. Advanced inorganic chemistry. New York: Wiley; 1988. p. 730.
Patel KN, Patel NH, Patel KM, Patel MN. Synthesis and characterization of cobalt(II), nickel(II), copper(II) and zinc(II) mixed-ligand complexes. Synth React Inorg Metal Org Chem. 2000;30(5):921–30.
Ferenc W, Cristvao B, Sarzynski J. Thermal and magnetic behavior of 5-chloro-2-nitrobenzoates of Co(II), Ni(II) and Cu(II). J Therm Anal. 2010;101(2):761–7.
Dziewulska-Kulaczkowska A. Manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II) complexes with 4-oxo-4H-1-benzopyran-3-carboxaldehyde: thermal, spectroscopic and magnetic characterization. J Therm Anal. 2010;101(3):1019–26.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B Polymer Lett. 1966;4(5):323–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(x):1881–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2(3):301–24.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6(x):183–95.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

10973_2011_1692_MOESM1_ESM.jpg
Fig. 1s XRD pattern of the thermal decomposition material at 1300 °C in N2, for compounds (1) [Co(3-OCH3-salo)2(neoc)] and/or (2) [Co(5-CH3-salo)2(neoc)]. (JPEG 105 kb)
Rights and permissions
About this article
Cite this article
Lalia-Kantouri, M., Gdaniec, M., Czapik, A. et al. Thermoanalytical, magnetic and structural study of Co(II) complexes with substituted salicylaldehydes and neocuproine. J Therm Anal Calorim 109, 131–139 (2012). https://doi.org/10.1007/s10973-011-1692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1692-5