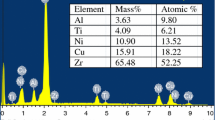
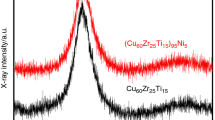
Abstract
Metallic glasses have received considerable attention in comparison to normal metallic materials due to their superior physical and mechanical properties. These systems possess large under cooled region, ∆T (∆T = T x − T g where, T x is crystallization temperature and T g is glass transition temperature) and hence increased thermal stability against crystallization. Due to this, the study of their crystallization kinetics is important and interesting. It is interesting because of the fact that, crystallization becomes multi-step process due to several components present in these systems. In this paper, we report the experimental investigations of crystallization of Zr52Cu18Ni14Al10Ti6 glassy alloy system, which is among the best non-beryllium containing glasses, using differential scanning calorimetry (DSC). The crystallization, as expected, consists of multiple steps. Interestingly, the peak heights of these steps vary with heating rate. At lower heating rates, first peak is most prominent and subsequently diminishes with increase in heating rate with last peak prominence visible at highest heating rate. Both, iso-kinetic and iso-conversional methods of analysis of kinetics of crystallization have been used to evaluate the activation energy and Avrami exponents and consistent results are obtained.













Similar content being viewed by others
References
Glade SC, Busch R, Lee DS, Johnson WL, Wunderlich RK, Fecht HJ. Thermodynamics of Cu47Ti34Zr11Ni8, Zr52.5Cu17.9Ni14.6Al10Ti5 and Zr57Cu15.4Ni12.6Al10Nb5 bulk metallic glass forming alloys. J Appl Phys. 2000;87:7242–8.
Ligero RA, Vazquez J, Villares P, Jimenez-Garay R. Crystalllization kinetics in the As–Se–Te system. Thermochim Acta. 1990;162:427–34.
Moharram AH, El-Oyoun MA, Abu-Sehly AA. Calorimetric study of the chalcogenide Se72.5Te20Sb7.5 glass. J Phys D Appl Phys. 2001;34:2541–6.
Rysava N, Spasov T, Tichy L. Isothermal DSC methods for evaluation of the kinetics of crystallization in the Ge–Sb–S glassy system. J Therm Anal. 1987;32:1015–21.
Giridhar A, Mahadevan S. Studies on the As–Sb–Se glass system. J Non Cryst Solids. 1982;51:305–15.
Afify S. Differential scanning calorimetric study of chalcogenide glass Se0.7Te0.3. J Non Cryst Solids. 1991;128:279–84.
Lad KN, Savalia RT, Pratap A, Dey GK, Banerjee S. Isokinetic and isoconversional study of crystallization kinetics of a Zr-based metallic glass. Thermochim Acta. 2008;473:74–80.
Kolmogorov AN. On the statistical theory of the crystallization of metals. Bull Acad Sci USSR Phys Ser. 1937;3:555.
Johnson WA, Mehl PA. Reaction kinetics of nucleation and growth. Trans Am Inst Min Metall Eng. 1939;135:416–32.
Avrami M. Kinetics of phase change. I General theory. J Chem Phys. 1939;7:1103–12.
Avrami M. Kinetics of phase change. II Transformations-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177–84.
Starink MJ. On the meaning of the impingement parameter in kinetic equations for nucleation and growth reactions. J Mater Sci. 2001;36:4433–41.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Res Report Chiba Inst Technol. 1971;16:22–31.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Boswell PG. On the calculation of activation energies using modified Kissinger method. J Therm Anal. 1980;18:353–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand A Phys Chem. 1966;70A:487–523.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6:642–93.
Doyle CD. Series approximations to the equation of thermogravimetric data. Nature. 1965;207:290–1.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to phenolic plastic. J Polym Sci. 1964;C6:183–95.
Gao YQ, Wang W. On the activation energy of crystallization in metallic glasses. J Non Cryst Solids. 1986;81:129–34.
Matusita K, Sakka S. Kinetic study of crystallization of glass by differential scanning calorimetry. Phys Chem Glasses. 1979;20:81–4.
Matusita K, Sakka S. Kinetic study on crystallization of glass by differential thermal analysis—criterion on application of Kissinger plot. J Non Cryst Solids. 1980;38–39:741–6.
Dhurandhar H, Patel AT, Shanker Rao TL, Lad KN, Pratap A. Kinetics of crystalllization of co-based multi-component amorphous alloy. J ASTM Int. 2010;7:1–15.
Sunol JJ, Bonastre J. Crystallization kinetics of metallic glasses. J Therm Anal Calorim. 2010;102:447–50.
Munteanu G, Segal E. Sestak–Berggren function in temperature-programmed reduction. J Therm Anal Calorim. 2010;101:89–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A.T., Pratap, A. Kinetics of crystallization of Zr52Cu18Ni14Al10Ti6 metallic glass. J Therm Anal Calorim 107, 159–165 (2012). https://doi.org/10.1007/s10973-011-1549-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1549-y