Abstract
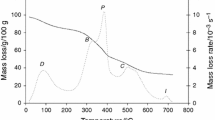
The Brazilian sugarcane industry shows a great amount of generated sludge which should be utilized adequately. Two sludge samples, aerobic and anaerobic, were collected. Both were evaluated by thermogravimetry and differential thermal analysis (DTA) as well as X-ray power diffraction. These compounds show variations of mass between 30 and 140 °C due to the dehydration stage. The DTA curves show that the compounds have an exothermic reaction between 450 and 550 °C, which indicates that this can be used as an energy source. Details concerning the kinetic parameters of the dehydration and thermal decomposition have also been described here. The kinetic study of these stages was evaluated in open crucibles under nitrogen atmosphere. The obtained data were evaluated with the isoconversional kinetic method. The results show that different activation energies were obtained for thermal decomposition.







Similar content being viewed by others
References
Associação Brasileira de Normas Técnicas. NBR 10.004—resíduos sólidos: classificação. Rio de Janeiro. 2004;71.
Nogueira TAR, Sampaio RA, Ferreira CS, Fonseca IM. Produtividade de milho e de feijão consorciados adubados com diferentes formas de lodo de esgoto. Rev Biol Ciên Terra. 2006;6:134–9.
Lopes JC, Ribeiro LG, Araújo MG, Beraldo MRBS. Produção de alface com doses de lodo de esgoto. Hortic Bras. 2005;23:143–7.
Oliveira FC, Mattiazzo ME, Marciano CR, Moraes SO. Lixiviação de nitrato em um latossolo amarelo distrófico tratado com lodo de esgoto e cultivado com cana-de-açúcar. Sci Agríc. 2001;58:171–80.
Menezes RR, Neves GA, Ferreira HC. O estado da arte sobre o uso de resíduos como matérias-primas cerâmicas alternativas. Rev Bras Eng Agríc Ambient. 2002;6:303–13.
Verstraete WBD, Pena M, Lettinga G, Lens P. Anaerobic bioprocessing of organic wastes. World J Microbiol Biotechnol. 1996;12:221–38.
Pinto CP. Tecnologia da Digestão Anaeróbia da Vinhaça e Desenvolvimento Sustentável. Dissertação. 1999.Universidade Estadual de Campinas (UNICAMP),Campinas, São Paulo.
Driessen W, Tielbaard M, Vereijken T. Experience on anaerobic treatment of distillery effluent with the UASB process. Water Sci Technol. 1994;30:193–201.
Driessen W, Habets L, Schouten T, Groeneveld N. New development in anaerobic reactor design for the treatment of industrial effluents. WISA Conference at Porth Elisabeth, South África. 1996; 20–23 May.
Kobelnik M, Bernabé GA, Ribeiro CA, Capela JMV, Fertonani FL. Kinetic of decomposition of iron (III)–diclofenac compound. J Therm Anal Calorim. 2009;97:493–6.
Souza JL, Kobelnik M, Ribeiro CA, Capela JMV. Kinetics study of crystallization of PHB in presence of hydroxy acids. J Therm Anal Calorim. 2009;97:525–8.
Kobelnik M, Cassimiro DL, Ribeiro CA, Dias DS, Crespi MS. Preparation of the Ca–diclofenac complex in solid state: study of the thermal behavior of the dehydration, transition phase and decomposition. J Therm Anal Calorim. 2010;102:1167–73.
Kobelnik M, Quarcioni VA, Ribeiro CA, Capela JMV, Dias DS, Crespi MS. Thermal behavior in solid state of Zn(II)–diclofenac complex: dehydration, phase transition and thermal decomposition. J Chin Chem Soc. 2010;57:384–90.
Kobelnik M, Ribeiro CA, Dias DS, Almeida S, Crespi MS, Capela JMV. Study of the thermal behavior of the transition phase of Co(II)–diclofenac compound by non-isothermal method. J Therm Anal Calorim. 2010. doi:10.1007/s10973-010-1208-8.
Kobelnik M, Cassimiro DL, Almeida AE, Ribeiro CA, Dias DS, Almeida S, Crespi MS. Study of the thermal behavior of Al(III) and In(III)–diclofenac complexes in solid state. J Therm Anal Calorim. 2010. doi:10.1007/s10973-010-1266-y.
Kobelnik M, Cassimiro DL, Dias DS, Ribeiro CA, Crespi MS. Thermal behavior of Jerivá oil (Syagrus romanzoffiana). J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1308-0.
Capela JMV, Capela MV, Ribeiro CA. Nonisothermal kinetic parameters estimated using nonlinear regression. J Math Chem. 2009;45:769–75.
Capana AS, Valentin QM, Crespi MS, Ribeiro CA, Barud HS. Thermal behavior of residues (sludge) originated from Araraquara water and sewage treatment station. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0361-4.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lourenço, R.V., Kobelnik, M., Ribeiro, C.A. et al. Thermal behavior of residues (sludge) originating from the sugarcane industry. J Therm Anal Calorim 106, 735–740 (2011). https://doi.org/10.1007/s10973-011-1396-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1396-x