Abstract
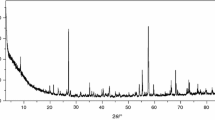
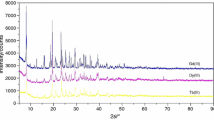
Solid-state compounds of general formula ThL4·nH2O, where L represents 2-methoxybenzylidenepyruvate and 2-methoxycynamylidenepyruvate, were synthesized. Complexometric titrations with EDTA, thermogravimetry (TG), differential thermal analysis (DTA), X-ray powder diffractometry, elemental analysis (EA), and infrared spectroscopy have been employed to characterize and to study the thermal behavior of these compounds in dynamic air atmosphere. The results led to informations about the composition, dehydration, crystallinity, and thermal decomposition of the isolated compounds. The performed molecular calculations in this study were done using the Gaussian 03 routine. Theoretical calculations help in interpretations of FT-IR spectra supplying structural and physicochemical parameters.


Similar content being viewed by others
References
Bannach G, Schnitzler E, Treu-Filho O, Utuni VHS, Ionashiro M. Synthesis, characterization and thermal studies on solid compounds of 2-chlorobenzylidenepyruvate of heavier trivalent lanthanides and yttrium (III). J Therm Anal Calorim. 2006;83:233–40.
Pereira NCS, Melios CB, Marques RN, Siqueira OS, Moraes M, Molina M, Ionashiro M. 4-dimethylaminocinnamylidenepyruvic acid: synthesis, characterization and complexation with trivalent lanthanides, yttrium (III), scandium (III), thorium (IV) and uranium (VI) in aqueous solution. J Alloys Compd. 1997;249:94–8. References there in.
Schnitzler E, Melios CB, Ionashiro M. Solid-state compounds of 4-methoxybenzylidenepyruvate and cinnamylidenepyruvates with thorium (IV) - Preparation and thermal studies. benzylidenepyruvate of heavier trivalent lanthanides and yttrium (III). J Therm Anal Calorim. 2002;70:581–92.
Bannach G, Mendes RA, Ionashiro EY, Mauro AE, Schnitzler E, Ionashiro M. Thermal studies on solid 2-chlorobenzylidenepyruvate of lighter trivalent lanthanides. J Therm Anal Calorim. 2005;79:329–34.
Ionashiro EY, Bannach G, Siqueira AB, Carvalho CT, Rodrigues EC. Ionashiro M 2-methoxybenzylidenepyruvate with heavier trivalent lanthanides and yttrium (III) synthesis and characterization. J Therm Anal Calorim. 2008;92:953–9.
Carvalho CT, Siqueira AB, Treu-Filho O, Ionashiro EY, Ionashiro M. Synthesis, characterization and thermal behaviour of solid 2-methoxycinnamylidenepyruvate of light trivalent lanthanides. J Braz Chem Soc. 2009;20:1313–9.
Dametto PR, Ambrozini B, Siqueira AB, Carvalho CT, Ionashiro M. Synthesis, characterization and thermal behaviour of solid-state 3-methoxybenzoates of heavy trivalent lanthanides and yttrium(III). J Therm Anal Calorim. 2009;101:933–9.
Siqueira AB, Carvalho CT, Rodrigues EC, Ionashiro EY, Bannach G, Ionashiro M. Synthesis, characterization and thermal behaviour of heavy lanthanide and yttrium pyruvates in the solid state. J Therm Anal Calorim. 2010;100:95–100.
Ionashiro M, Graner CAF, Zuanon-Netto J. Titulação complexométrica de lantanídeos e Ítrio. Ecl Quim. 1983;8:29–32.
Becke AD. Density-functional thermochemistry. 3. The role of exact exchange. J Chem Phys. 1993;98:5648–52.
Lee C, Yang W, Parr RG. Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B. 1988;37:785–9.
Treu-Filho O, Pinheiro JC, da Costa EB, Ferreira JEV, de Figueiredo AF, Kondo RT, de Lucca Neto VA, de Souza RA, Legendre AO, Mauro AE. Experimental and theoretical study of the compound [Pd(dmba)(NCO)(imz)]. J Mol Struct. 2007;829:195–201.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA. Gaussian 03, Revision B.04. Pittsburgh: Gaussian, Inc; 2003.
Schelegel HB. In: Bertran J, editor. New theoretical concepts for understanding organic reactions. The Netherlands: Academic; 1989. p. 33–53.
Goodson DZ, Sarpal SK, Bopp P, Wolfsberg M. Influence on isotope effect calculations of the method of obtaining force constants from vibrational data. J Phys Chem. 1982;86:659–63.
Roy Dennington II, Keith Todd, Millam John, Ken Eppinnett W, Hovell Lee, Gilliland Ray. Gaussview, version 3.0. Shawnee Mission: Semichem, Inc.; 2003.
Deacon GB, Phillips RJ. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. J Coord Chem. 1980;33:227–50.
Acknowledgements
The authors are very grateful for the financial support by CAPES, CNPq, and FAPESP. The computational facilities were employed at IQ-UNESP-Ar and CENAPAD-UNICAMP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schnitzler, E., Bannach, G., Treu-Filho, O. et al. Solid-state compounds of 2-methoxybenzylidenepyruvate and 2-methoxycinnamyllidenepyruvate with thorium (IV). J Therm Anal Calorim 106, 643–649 (2011). https://doi.org/10.1007/s10973-010-1262-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1262-2