Abstract
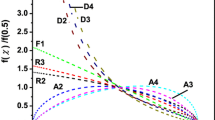
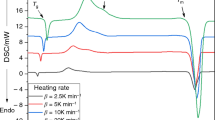
Carrying out crystallization studies for both Se0.95In0.05 and Se0.90In0.10 chalcogenide glasses under non-isothermal conditions at different heating rates, it was realized that a rate controlling process occurs where random nucleation of one- to two-dimensional growth is accompanied with the introduction of up to 10 at% In into glassy Se matrix. The crystallization kinetics together with its dimensionality has been studied using the four currently used isoconversional models (Kissinger–Akahira–Sunose, Ozawa–Flynn–Wall, Tang, and Starink). The activation energy of crystallization (E c) has been determined using these indicated four models where a satisfactory concurrence is achieved. The value of E c shows a decrease while increasing both the In-content as well as the extent of crystallization.









Similar content being viewed by others
References
Mott NF, Davis EA. Electronic processes in non-crystalline materials. Oxford: Clarendon Press; 1971.
Kotkata MF. Transports and structural properties of non-crystalline chalcogenide semiconductors. D.Sc. Thesis, Hungarian Academy of Sciences: Budapest, Hungary; 1993.
Mehta N, Kumar A. Comparative analysis of calorimetric studies in Se90M10 (M = In, Te, Sb) chalcogenide glasses. J Therm Anal Calorim. 2007;87:343–8.
Sharma A, Barman PB. Effect of Bi incorporation on the glass transition kinetics of Se85Te15 glassy alloy. J Therm Anal Calorim. 2009;96:413–7.
Kotkata MF, Abdel-Wahab FA, Al-Kotb MS. Effect of In-content on the optical properties of a-Se films. Appl Surf Sci. 2009;255:9071–7.
Shukla S, Kumar S. Effect of Sb incorporation on the dark conductivity and photoconductivity of Se75In25 glassy alloy thin films. Physica B. 2010;405:4307–12.
Kotkata MF, Mansour ShA. Study of glass transition kinetics of selenium matrix alloyed with up to 10% indium. J. Therm Anal Calorim. 2010; doi:10.1007/s10973-010-0963-x.
Avrami M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Kotkata MF, Kamal GM, El-Mously MK. Electrical conductivity and crystallization of amorphous selenium doped with tellurium. Indian J Technol. 1982;20:390–5.
Hay JN. Application of the modified Avrami equations to polymer crystallization kinetics. Br Polym J. 1971;3:74–82.
Henderson DW. Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non-Cryst Solids. 1979;30:301–15.
Lopez-Alemany PL, Vazquez J, Villares P, Jimenez-Garay R. Theoretical analysis on the mechanism and transformation kinetics under non-isothermal conditions application to the crystallization of semiconducting Sb0.16As0.36Se0.48 alloy. Mater Chem Phys. 2000;65:150–7.
Kotkata MF, Mahmoud EA. Non-isothermal crystallization kinetic studies on amorphous chalcogenide semiconductors. Mater Sci Eng. 1982;54:163–8.
Brown ME, Dollomore D, Galwey AK. Reaction in solid state. Comprehensive chemical kinetics. 22nd ed. Amsterdam: Elsevier; 1980.
Sharp JH, Brindley GW, Narahari Achar BN. Numerical data for some commonly used solid state reaction equations. J Am Ceram Soc. 1966;49:379–82.
Ozawa T. Kinetics of non-isothermal crystallization. Polymer. 1971;12:150–8.
Kotkata MF. Trends in the microhardness of mono-component and multi-component chalcogenide glasses. J Mater Sci. 1991;26:4869–77.
Šimon P. Isoconversional methods. Fundamentals, meaning and application. J Therm Anal Calorim. 2004;76:123–32.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Joint convention of four electrical institutes. Res Report Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Japan. 1965;38:1881–6.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4:323–8.
Tang W, Lui Y, Zhang H, Wang C. New approximation formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Coats AW, Redfern JP. Parameters from thermogravimetric data. Nature. 1964;201:68–9.
Doyle CD. Series approximations to the equations of thermogravimetric data. Nature. 1965;207:290–1.
Kotkata MF. Phase property study of semiconductor selenium: part I. J Mater Sci. 1992;27:4847–57.
Shukla R, Agarwal P, Kumar A. Crystallization kinetics in glassy Se100−x In x system using isoconversional methods. Chalcogenide Lett. 2010;7:249–55.
Pacurariu C, Lazau RI, Lazau I, Lanos R, Vlase T. Influence of the specific surface area on crystallization process kinetics of some silica gels. J Therm Anal Calorim. 2009;97:409–14.
Biswas K, Sontakk AD, Majumder M, Annapurna K. Non-isothermal crystallization kinetics and microstructure evolution of calcium lanthanum metaborate glass. J Therm Anal Calorim. 2010;101:143–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotkata, M.F., Mansour, S.A. Crystallization process analysis for Se0.95In0.05 and Se0.90In0.10 chalcogenide glasses using the contemporary isoconversional models. J Therm Anal Calorim 103, 957–965 (2011). https://doi.org/10.1007/s10973-010-1120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1120-2