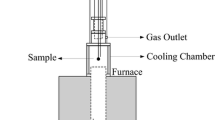
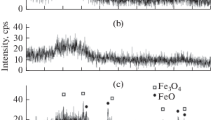
Abstract
In this study, we have attempted to explain the complex reactions that occur during the dehydration of Ca(OH)2, in the presence of solid carbon and Fe2O3, in order to clarify their role as eventual precursors to the reduction and high temperature strength characteristics in feedstock agglomerates of iron and steelmaking by-products. A series of simultaneous thermo-gravimetric (TG), differential thermal analytic (DTA), and mass spectrometric (MS) tests were performed on agglomerated sample mixes of Ca(OH)2, C, and Fe2O3 to test the influence of heating rate and particle size on the transformations occurring below 1,073 K in inert atmosphere. The overall transformation begins with calcium hydroxide dehydration. Nucleation and growth of CaO grains during dehydration, as well as subsequent gasification of solid carbon, are highly dependent on the governing interstitial particle porosity and mildly dependent on the heating rate in and around agglomerates. The reduction of hematite in current agglomerates is, by association to preceding reactions, partly dependent on porosity and heating rate, but the mechanism of reduction was also found to be highly dependent on the particle size of iron oxides. Furthermore, in areas of intimate contact between CaO and iron oxide, a calcium ferrite phase appears in the form of angular and calcium-rich particles.








Similar content being viewed by others
References
Takano C, Nascimento RC, Silva GFBL, dos Santos DM, Mourao MB. Recycling of solid wastes containing iron from integrated steelmaking plant. In: EPD congress; 2001, TMS, p. 183–193.
Gudenau HW, Senk D, Wang S, De Melo Martins K, Stephany C. Research in the reduction of iron ore agglomerates including coal and C containing dust. ISIJ Int. 2005;45(4):603–8.
Ahn JS, Chon CM, Moon HS, Kim KW. Arsenic removal using steel manufacturing byproducts as permeable reactive materials in mine tailing containment system. Water Res. 2003;37(10):2478–88.
Robinson R. High temperature properties of by-product cold bonded pellets containing blast furnace flue dust. Thermochim Acta. 2005;432:112–23.
Takano C, Mourao MB. Self-reducing pellets for ironmaking: mechanical behavior. Miner Processing Extr Metall Rev. 2003;24:233–52.
Mantovani MC, Takano C. The strength and high temperature behaviors of self-reducing pellets containing EAF dust. ISIJ Int. 2000;40(3):224–30.
Kashiwaya Y, Kanbe M, Shii K. Reaction behavior of facing pair between hematite and graphite: a coupling phenomenon of reduction and gasification. ISIJ Int. 2001;41:818.
Mookherjee S, Ray HS, Mukhrjee A. Isothermal reduction of iron ore fines surrounded by coal och char fines. Ironmaking Steelmaking 1986;13:229–35.
Lu W-K, Bryk C, Gou H. The LB furnace for smelting reduction of iron ore. In: Proceedings of the 5th international iron and steel congress, Book 3, vol 6. 1986, p. 1065–1075.
Gou H, Lu W-K, Bryk C. Bench scale test of a new ironmaking process with mixture of iron ore concentrate and pulverized coal. ISIJ Int. 1992;32:733–40.
Kumabe K, Moritomi H, Yoshiie R, Kambara S, Kuramoto K, Suzuki Y, Hatano H, Lin SY, Harada M. Gasification of organic waste with subcritical steam under the presence of a calcium-based carbon dioxide sorbent. Ind Eng Chem Res. 2004;43:6943–7.
Wang J, Takarada T. Role of calcium hydroxide in supercritical water gasification of low-rank coal. Energy Fuels. 2001;15:356–62.
Inui T, Otowa T, Okazumi F. Gasification of active carbon by iron-based composite catalysts for obtaining directly a gas of optional H2/CO ratio. Carbon. 1985;23(2):193–208.
Sato S, Lin SY, Suzuki Y, Hatano H. Hydrogen production from heavy oil in the presence of calcium hydroxide. Fuel. 2003;82:561–7.
Sohn HY, Wadsworth EM. Rate processes of extractive metallurgy. New York: Plenum Press; 1979.
Robinson R, Menad N, Björkman B. Low temperature behavior of the Ca(OH)2–C system and its significance on the self-reduction of cold bonded by-product agglomerates. In: Proceedings of the 4th ICSTI. 2006, p. 311–314.
Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J Therm Anal Calorim. 2000;60:641–58.
Ortega A. The kinetics of solid-state reactions toward consensus, Part 2: fitting kinetic data in dynamic conventional thermal analysis. Int J Chem Kinetics. 2002;34:193–208.
Koga N, Sestak J, Malek J. Distortion of the Arrhenius parameters by the inappropriate kinetic model function. Thermochim Acta. 1991;188:333.
Criado JM, Ortega A, Gotor F. Correlation between the shape of controlled rate thermal analysis curves and the kinetics of solid state reactions. Thermochim Acta. 1990;157:171–9.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Galwey AK, Laverty GM. A kinetic and mechanistic study of the dehydroxylation of calcium hydroxide. Thermochim Acta. 1993;228:359–78.
Koga N, Tanaka H. A physico-geometric approach to the kinetics of solid-state reactions as exemplified by the thermal dehydration and decomposition of inorganic solids. Thermochim Acta. 2002;388:41–61.
Barret P. In: International proceedings of the 4th symposium on the reactivity of solids. 1960, p. 178.
Mann MD, Knutson RZ, Erjavec J, Jacobsen JP. Modelling reaction kinetics of steam gasification for a transport gasifier. Fuel. 2004;83:1643–50.
El-Geassy AHA. Stepwise reduction of CaO and/or MgO doped-Fe2O3 compacts (hematite-wustite-iron transformation steps). Scandinavian J Metall. 1998;27:205.
Turkdogan ET. Reduction of iron oxides. In: 31st Ironmaking conference proceedings. 1972, p. 438–458.
Turkdogan ET, Vinters JV. Gaseous reduction of iron oxides: Part I. Reduction of hematite in hydrogen. Metall Mater Trans B. 1971;2(11):3175–88.
Dutta SK, Ghosh A. Kinetics of gaseous reduction of iron ore fines. ISIJ Int. 1993;33:1168–73.
Fortini OM, Fruehan RJ. Rate of reduction of ore-carbon composites: Part II. Modeling of reduction in extended composites. Metall Mater Trans B. 2005;36(B):709–17.
Haque R, Ray HS. Role of ore/carbon contact and direct reduction in the reduction of iron oxide by carbon. Metall Mater Trans B. 1995;26(B):400–1.
Malek J. The applicability of Johnson–Mehl–Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim Acta. 1995;267:61–73.
Turkdogan ET, Vinters JV. Effect of carbon monoxide on the rate of oxidation of charcoal, graphite and coke in carbon dioxide. Carbon. 1970;8:39–53.
Acknowledgements
The authors would like to express their gratitude to Mr. Bernard Rouat for all help in laboratory and the Foundation of King Carl XVI Gustaf’s 50th Birthday Fund for financial support during the completion of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, R., Patisson, F. & Björkman, B. Low temperature reactivity in agglomerates containing iron oxide. J Therm Anal Calorim 103, 185–193 (2011). https://doi.org/10.1007/s10973-010-1059-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1059-3