Abstract
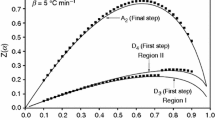
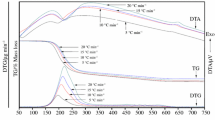
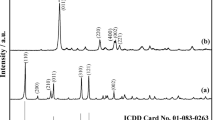
The single phase NH4NiPO4·6H2O was synthesized by solid-state reaction at room temperature using NiSO4·6H2O and (NH4)3PO4·3H2O as raw materials. XRD analysis showed that NH4NiPO4·6H2O was a compound with orthorhombic structure. The thermal process of NH4NiPO4·6H2O experienced three steps, which involves the dehydration of the five crystal water molecules at first, and then deamination, dehydration of the one crystal water, intramolecular dehydration of the protonated phosphate groups together, at last crystallization of Ni2P2O7. In the DTA curve, the two endothermic peaks and an exothermic peak, respectively, corresponding to the first two steps’ mass loss of NH4NiPO4·6H2O and crystallization of Ni2P2O7. Based on Flynn–Wall–Ozawa equation, and Kissinger equation, the average values of the activation energies associated with the thermal decomposition of NH4NiPO4·6H2O, and crystallization of Ni2P2O7 were determined to be 47.81, 90.18, and 640.09 kJ mol−1, respectively. Dehydration of the five crystal water molecules of NH4NiPO4·6H2O, and deamination, dehydration of the crystal water of NH4NiPO4·H2O, intramolecular dehydration of the protonated phosphate group from NiHPO4 together could be multi-step reaction mechanisms. Besides, the thermodynamic parameters (ΔH ≠, ΔG ≠, and ΔS ≠) of the decomposition reaction of NH4NiPO4·6H2O were determined.






Similar content being viewed by others
References
Goñi A, Pizarro JL, Lezama LM, Barberis GE, Arriortua MI, Rojo T. Synthesis, crystal structure and spectroscopic properties of the NH4NiPO4·nH2O (n = 1, 6) compounds; magnetic behaviour of the monohydrated phase. J Mater Chem. 1996;6:421–7.
Li YF, Cui W, Zhu GS, Qiu SL, Fang QR, Wang CL. Hydrothermal synthesis and characterization of Ni3(PO4)2·8H2O with 8-ring and 4-ring network structure. Chem J Chin Univ. 2002;23:1480–2.
Wu WW, Fan YJ, Wu XH, Liao S, Li SS. Preparation via solid-state reaction at room temperature and characterization of layered nanocrystalline NH4MnPO4·H2O. J Phys Chem Solids. 2009;70:584–7.
Koleva VG. Metal–water interactions and hydrogen bonding in dittmarite-type compounds M′M′′PO4·H2O(M′ = K+, NH4 +; M′′ = Mn2+, Co2+, Ni2+)—Correlations of IR spectroscopic and structural data. Spectrochim Acta A. 2005;62:1196–202.
Carling SG, Day P, Visser D. Crystal and magnetic structures of layer transition metal phosphate hydrates. Inorg Chem. 1995;34:3917–27.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Ozawa TA. New method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Boonchom B, Puttawong S. Thermodynamics and kinetics of the dehydration reaction of FePO4·2H2O. Phys B. 2010;405:2350–5.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrol. 2008;81:253–62.
Rajić N, Ristić A, Kaučič V. On the possibility of the preparation open framework manganese phosphate. Zeolites. 1996;17:304–9.
Boonchom B, Danvirutai C, Santi Maensiri S. Soft solution synthesis, non-isothermal decomposition kinetics and characterization of manganese dihydrogen phosphate dihydrate Mn(H2PO4)2·2H2O and its thermal transformation. Mater Chem Phys. 2008;109:404–10.
Onoda H, Sugino N, Kojima K, Nariai H. Mechanochemical effects on synthesis and properties of manganese–neodymium diphosphates. Mater Chem Phys. 2003;82:831–6.
Šoptrajanov B, Jovanovski G, Pejov L. Very low H–O–H bending frequencies. III. Fourier transform infrared study of cobalt potassium phosphate monohydrate and manganese potassium phosphate monohydrate. J Mol Struct. 2002;613:47–54.
Wu XX, Wu WW, Liao S, Fan YJ, Li SS. Preparation via solid-state reaction at room temperature and characterization of layered nanocrystalline KMnPO4·H2O. J Alloys Compd. 2009;479:541–4.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Budrugeac P, Muşat V, Segal E. Non-isothermal kinetic study on the decomposition of Zn acetate-based sol-gel precursor. J Therm Anal Calorim. 2007;88:699–702.
Boonchom B, Danvirutai C. Kinetics and thermodynamics of thermal decomposition of synthetic AlPO4·2H2O. J Therm Anal Calorim. 2009;98:771–7.
Boonchom B. Kinetics and thermodynamic properties of the thermal decomposition of manganese dihydrogenphosphate dihydrate. J Chem Eng Data. 2008;53:1533–8.
Danvirutai C, Noisong P, Youngme S. Some thermodynamic functions and kinetics of thermal decomposition of NH4MnPO4·H2O in nitrogen atmosphere. J Therm Anal Calorim. 2010;100:117–24.
Acknowledgements
This study was financially supported by the Guangxi Natural Scientific Foundation of China (Grant No. 0832111), and the Guangxi Science and Technology Agency Research Item of China (Grant No. 0895002–9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Wu, W., Li, S. et al. Kinetics and thermodynamics of thermal decomposition of NH4NiPO4·6H2O. J Therm Anal Calorim 103, 805–812 (2011). https://doi.org/10.1007/s10973-010-1057-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1057-5