Abstract
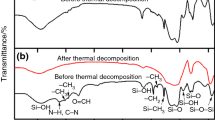
The phase transition at T p (~109 °C) of RbH2PO4 and its composite with SiO2 has been investigated by thermal analysis here. In the case of neat RbH2PO4, there is a linear relationship between endothermic peak temperature (T m) and square root of heating rate (Φ 1/2), from which the onset temperature of phase transition can be determined. Besides, Kissinger method and another calculation method were employed to obtain the activation energy of phase transition. The detailed deduction process was presented in this paper, and the estimated activation energies are E 1 ≈ 126.3 kJ/mol and E 2 ≈ 129.2 kJ/mol, respectively. On the other hand, the heterogeneous doping of RbH2PO4 with SiO2 as dopant facilitates its proton conduction and leads to the disappearance of jump in conductivity at T p. The heats of transition in the composites decrease gradually with increasing the molar fraction of SiO2 additives. In the cooling process, a new and broad exothermic peak appeared between ~95 and ~110 °C, and its intensity also changes with the SiO2 amount. These phenomena might be related to the formation of amorphous phase of RbH2PO4 on the surface of SiO2 particles due to the strong interface interaction.








Similar content being viewed by others
References
Haile SM, Boysen DA, Chisholm CRI, Merle RB. Solid acids as fuel cell electrolytes. Nature. 2001;410:910–3.
Boysen DA, Uda T, Chisholm CRI, Haile SM. High-performance solid acid fuel cells through humidity stabilization. Science. 2004;303:68–70.
Metcalfe B, Clark JB. Differential scanning calorimetry of RbH2PO4 and CsH2PO4. Thermochim Acta. 1978;24:149–53.
Blinc R, O’Reilly DE, Peterson EM, Williams JM. High-temperature phase transition in RbH2PO4. J Chem Phys. 1969;50:5408–11.
Blinc R, Ferraro JR, Postmus C. Effects of high pressure on the far-infrared spectra of paraelectric KH2PO4 and RbH2PO4. J Chem Phys. 1969;51:732–7.
Averbuch-Pouchot MT, Durif A. Structure of a new form of rubidium dihydrogen phosphate, RbH2PO4. Acta Crystallogr C. 1985;41:665–7.
Baranov AI, Khiznichenko VP, Shuvalov LA. High-temperature phase transition and proton conductivity in some KDP-family crystals. Ferroelectrics. 1989;100:135–41.
Boysen DA, Haile SM. Conductivity of potassium and rubidium dihydrogen phosphates at high temperature and pressure. Chem Mater. 2004;16:693–7.
Lee KS. Hidden nature of the high-temperature phase transition in crystals of KH2PO4-type: is it a phase change? J Phys Chem Solids. 1996;57:333–42.
Ortiz E, Vargas RA, Cuervo G, Mellander BE, Gustafson J. On the high-temperature phase transition of RbH2PO4—a polymorphic transition? J Phys Chem Solids. 1998;59:1111–7.
Ortiz E, Vargas RA, Mellander BE. On the high-temperature phase transitions of some KDP-family compounds: a structural phase transition? A transition to a bulk-high proton conducting phase? Solid State Ion. 1999;125:177–85.
Park JH, Lee KS, Choi BC. High-temperature transformation in KH2PO4 and RbH2PO4 crystals. J Phys Condens Matter. 2001;13:9411–9.
Park JH. Possible origin of the proton conduction mechanism of CsH2PO4 crystals at high temperatures. Phys Rev B. 2004;69:0541041–6.
Ponomareva VG, Uvarov NF, Lavrova GV, Hairetdinov EF. Composite protonic solid electrolytes in the CsHSO4-SiO2 system. Solid State Ion. 1996;90:161–6.
Ponomareva VG, Lavrova GV. Influence of dispersed TiO2 on protonic conductivity of CsHSO4. Solid State Ion. 1998;106:137–41.
Ponomareva VG, Lavrova GV, Simonova LG. The influence of heterogeneous dopant porous structure on the properties of protonic solid electrolyte in the CsHSO4–SiO2 system. Solid State Ion. 1999;118:317–23.
Li ZK. Impedance analysis and protonic conduction mechanism in RbH2PO4/SiO2 composite systems. Electrochim Acta. Accepted for publication.
Li ZK, Tang TB. High-temperature thermal behaviors of XH2PO4 (X = Cs, Rb, K, Na) and LiH2PO3. Thermochim Acta. 2010;501:59–64.
Boysen DA, Haile SM. High-temperature behavior of CsH2PO4 under both ambient and high pressure conditions. Chem Mater. 2003;15:727–36.
Sanchez-Jimenez PE, Criado JM, Perez-Maqueda LA. Kissinger kinetic analysis of data obtained under different heating schedules. J Therm Anal Calorim. 2008;94:427–32.
Uvarov NF, Vanek P. Stabilization of new phases in ion-conducting nanocomposites. J Mater Synth Process. 2000;8:319–26.
Uvarov NF, Bokhonov BB, Politov AA, Vanek P, Petzelt J. Interface-stabilized states of silver iodide in AgI–Al2O3 composites. J Mater Synth Process. 2000;8:327–32.
Acknowledgements
The authors are very grateful to Prof. Tongbor Tang for his kind help. This work is supported financially by the Research Grant Council of Hong Kong (HKBU 210907).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Chan, W.E. Phase transition of RbH2PO4 and its composite with SiO2 studied by thermal analysis. J Therm Anal Calorim 104, 585–592 (2011). https://doi.org/10.1007/s10973-010-1005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1005-4