Abstract
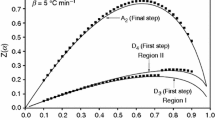
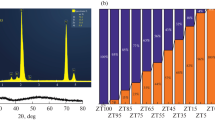
Nanocrystalline NH4ZrH(PO4)2·H2O was synthesized by solid-state reaction at low heat using ZrOCl2·8H2O and (NH4)2HPO4 as raw materials. X-ray powder diffraction analysis showed that NH4ZrH(PO4)2·H2O was a layered compound with an interlayer distance of 1.148 nm. The thermal decomposition of NH4ZrH(PO4)2·H2O experienced four steps, which involves the dehydration of the crystal water molecule, deamination, intramolecular dehydration of the protonated phosphate groups, and the formation of orthorhombic ZrP2O7. In the DTA curve, the three endothermic peaks and an exothermic peak, respectively, corresponding to the first three steps' mass losses of NH4ZrH(PO4)2·H2O and crystallization of ZrP2O7 were observed. Based on Flynn–Wall–Ozawa equation and Kissinger equation, the average values of the activation energies associated with the NH4ZrH(PO4)2·H2O thermal decomposition and crystallization of ZrP2O7 were determined to be 56.720 ± 13.1, 106.55 ± 6.28, 129.25 ± 4.32, and 521.90 kJ mol−1, respectively. Dehydration of the crystal water of NH4ZrH(PO4)2·H2O could be due to multi-step reaction mechanisms: deamination of NH4ZrH(PO4)2 and intramolecular dehydration of the protonated phosphate groups from Zr(HPO4)2 are simple reaction mechanisms.




Similar content being viewed by others
References
Parida KM, Sahu BB, Das DP. A comparative study on textural characterization: cation-exchange and sorption properties of crystalline α-zirconium(IV), tin(IV), and titanium(IV) phosphates. J Colloid Interface Sci. 2004;270:436–45.
Costa MCC, Hodson LF, Johnstone RAW, Junyao L, Whittaker D. The mechanism of gas-phase dehydration of cyclohexanol and the methylcyclohexanols catalysed by zirconium phosphate and zirconium phosphite. J Mol Catal A Chem. 1999;142:349–60.
Rustum R, Vance ER, Alamo J. [NZP], a new radiophase for ceramic nuclear waste forms. Mater Res Bull. 1982;17:585–9.
Krishna RM, Kevan L. Photoinduced electron transfer from N,N,N′,N′-tetramethylbenzidine incorporated into layered zirconium phosphate studied by ESR and diffuse reflectance spectroscopies. Micropor Mesopor Mater. 1999;32:169–74.
Clearfield A, Stynes JA. The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour. J Inorg Nucl Chem. 1964;26:117–29.
Carrière D, Moreau M, Lhalil K, Barboux P, Boilot JP. Proton conductivity of colloidal nanometric zirconium phosphates. Solid State Ionics. 2003;162–163:185–90.
Alberti G. Syntheses, crystalline structure and ion-exchange properties of insoluble acid salts of tetravalent metals and their salt forms. Acc Chem Res. 1978;11:163–70.
Tarafdar A, Panda AB, Pradhan NC, Pramanik P. Synthesis of spherical mesostructured zirconium phosphate with acidic properties. Micropor Mesopor Mater. 2006;95:360–5.
Yamanaka S, Yoshioka K, Hattori M. Unusual ionic conductivities of hydrothermally prepared MZr2(PO4)3 (M = Na, K). Solid State Ionics. 1990;40–41:43–7.
Wu WW, Lai SB, Wu XH, Liao S, Hou SY. Preparation of NH4ZrH(PO4)2 H2O via solid-state reaction at low heat and study on catalytic synthesis of butyl acetate. Rare met. 2008;27:550–4.
Logvinenko V. Solid state coordination chemistry. The quantitative thermoanalytical study of thermal dissociation reactions. J Therm Anal Calorim. 2000;60:9–15.
Danvirutai C, Noisong P, Youngme S. Some thermodynamic functions and kinetics of thermal decomposition of NH4MnPO4 H2O in nitrogen atmosphere. J Therm Anal Calorim. 2010;100:117–24.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4 3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98:863–71.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Ozawa TA. New method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Wu XH, Wu WW, Liao S, Fan YJ, Li SS. Preparation via solid-state reaction at room temperature and characterization of layered nanocrystalline KMnPO4 H2O. J Alloys Compd. 2009;479:541–4.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolysis. 2008;81:253–62.
Gabal MA. Non-isothermal decomposition of NiC2O4–FeC2O4 mixture aiming at the production of NiFe2O4. J Phys Chem Solids. 2003;64:1375–85.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Budrugeac P, Muşat V, Segal E. Non-isothermal kinetic study on the decomposition of Zn acetate-based sol-gel precursor. J Therm Anal Calorim. 2007;88:699–702.
Boonchom B, Danvirutai C. Kinetics and thermodynamics of thermal decomposition of synthetic AlPO4 2H2O. J Therm Anal Calorim. 2009;98:771–7.
Acknowledgements
This study was financially supported by the Guangxi Natural Scientific Foundation of China (Grant No. 0991108), and the Guangxi Science and Technology Agency Research Item of China (Grant No. 0992001-5).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Wu, X., Lai, S. et al. Non-isothermal kinetics of thermal decomposition of NH4ZrH(PO4)2·H2O. J Therm Anal Calorim 104, 685–691 (2011). https://doi.org/10.1007/s10973-010-0986-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0986-3