Abstract
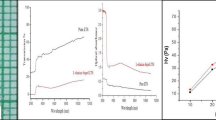
The influence of heteroaromatic N-base (1,10-phenanthroline) (Phen), a new additive as complexing agent on tris(thiourea)zinc(II)sulphate (ZTS) crystals from aqueous solutions at 30 °C is investigated. Crystals were grown using low concentration of the dopant (0.005 M L−1) in the aqueous growth medium and the growth promoting effect (GPE) is much greater because of an increase in the metastable zone width. High dopant concentration decreases GPE. The crystalline perfection of the grown crystals is quite good both in doped and undoped crystals as evaluated by high-resolution X-ray diffractometry (HRXRD). The diffraction curve of a typical Phen doped as-grown ZTS crystal was observed to contain a single peak indicating that the crystal does not contain any epitaxial layer on the surface or internal structural grain boundaries. Not much variation is observed in FT-IR and XRD of pure and doped ZTS. Phen depresses the NLO efficiency of ZTS. It could be ascribed due to the disturbance of charge transfer in the presence of the dopant. The grown crystals were also characterized by UV–Vis, SEM and TG–DTA techniques.





Similar content being viewed by others
References
Meenakshisundaram S, Parthiban S, Sarathi N, Kalavathy R, Bhagavannarayana G. Effect of organic dopants on ZTS single crystals. J Cryst Growth. 2006;293:376–81.
Verma S, Singh MK, Wadhavan VK, Suresh CH. Growth morphology of zinctris(thiourea)sulphate crystals. J Phys. 2000;54:879–88.
Gupte SS, Desai CF. Vickers hardness anisotropy and slip system in zinc(tris)thioureasulphate crystals. Cryst Res Technol. 1999;34:1329–32.
Venkataramanan V, Dhanaraj G, Wadhawan WK, Sherwood JN, Bhat HL. Crystal growth and defects characterization of zinctris(thiourea)sulfate: a novel metalorganic nonlinear optical crystal. J Cryst Growth. 1995;154:92–7.
Ushasree PM, Muralidharan R, Jayavel R, Ramasamy P. Metastable zone width, induction period and interfacial energy of zinctris(thiourea)sulfate. J Cryst Growth. 2000;210:741–5.
Arunmozhi G, de Gomes EM, Ganesamoorthy S. Growth kinetics of zinc(tris)thioureasulphate (ZTS) crystals. Cryst Res Technol. 2004;39:408–13.
Sangwal K, Mielniczek-Brzoska E. Effect of impurities on metastable zone width for the growth of ammonium oxalate monohydrate crystals from aqueous solutions. J Cryst Growth. 2004;267:662–75.
Li G, Xue L, Su G, Li Z, Zhuang X, He Y. Rapid growth of KDP crystal from aqueous solutions with additives and its optical studies. Cryst Res Technol. 2005;40:867–70.
Czakis-Sulikowska D, Czylkowska A, Malinowska A. Thermal and other properties of new 4, 4′-bipyridinetrichloroacetato complexes of Mn(II), Ni(II) and Zn(II). J Therm Anal Calorim. 2002;67:667–78.
More A, Verenkar VMS, Mojumdar SC. Nickel ferrite nanoparticles synthesis from novel fumarato-hydrazinate precursor. J Therm Anal Calorim. 2008;94:63–7.
Mojumdar SC, Raki L. Preparation, thermal, spectral and microscopic studies of calcium silicate hydrate-poly(acrylic acid) nanocomposite materials. J Therm Anal Calorim. 2006;85:99–105.
Sawant SY, Verenkar VMS, Mojumdar SC. Preparation, thermal, XRD, chemical and FT-IR spectral analysis of NiMn2O4 nanoparticles and respective precursor. J Therm Anal Calorim. 2007;90:669–72.
Porob RA, Khan SZ, Mojumdar SC, Verenkar VMS. Synthesis, TG, SDC and infrared spectral study of NiMn2(C4H4O4)3·6N2H4—a precursor for NiMn2O4 nanoparticles. J Therm Anal Calorim. 2006;86:605–8.
Mojumdar SC, Varshney KG, Agrawal A. Hybrid fibrous ion exchange materials: past, present and future. Res J Chem Environ. 2006;10:89–103.
Doval M, Palou M, Mojumdar SC. Hydration behaviour of C2S and C2AS nanomaterials, synthesized by sol-gel method. J Therm Anal Calorim. 2006;86:595–9.
Varshney KG, Agrawal A, Mojumdar SC. Pyridine based thorium(IV) phosphate hybrid fibrous ion exchanger: synthesis, characterization and thermal behaviour. J Therm Anal Calorim. 2007;90:721–4.
Madhurambal G, Ramasamy P, Anbusrinivasan P, Mojumdar SC. Thermal properties, induction period, interfacial energy and nucleation parameters of solution grown benzophenone. J Therm Anal Calorim. 2007;90:673–9.
Varshney G, Agrawal A, Mojumdar SC. Pyridine based cerium(IV) phosphate hybrid fibrous ion exchanger: Synthesis, characterization and thermal behaviour. J Therm Anal Calorim. 2007;90:731–4.
Mojumdar SC, Melnik M, Jona E. Thermal and spectral properties of Mg(II) and Cu(II) complexes with heterocyclic N-donor ligands. J Anal Appl Pyrolysis. 2000;53:149–60.
Borah B, Wood JL. Complex hydrogen bonded cations. The benzimidazole benzimidazolium cation. Can J Chem. 1976;50:2470–81.
Mojumdar SC, Sain M, Prasad RC, Sun L, Venart JES. Selected thermoanalytical methods and their applications from medicine to construction. J Therm Anal Calorim. 2007;60:653–62.
Meenakshisundarm SP, Parthiban S, Madhurambal G, Mojumdar SC. Effect of chelating agent (1, 10-phenanthroline) on potassium hydrogen phthalate crystals. J Therm Anal Calorim. 2008;94:21–5.
Skorsepa JS, Gyoryova K, Melnik M. Preparation, identification and thermal properties of (CH3CH2COO)2Zn·2L·H2O (L = thiourea, nicotinamide, caffeine or theorbromine). J Therm Anal Calorim. 1995;44:169–77.
Ondrusova D, Jona E, Simon P. Thermal properties of N-ethyl-N-phenyldithiocarbamates and their influence on the kinetics of cure. J Therm Anal Calorim. 2002;67:147–52.
Kubranova M, Jona E, Rudinska E, Nemcekova K, Ondrusova D, Pajtasova M. Thermal properties of Co-, Ni- and Cu-exchanged montmorillonite with 3-hydroxypyridine. J Therm Anal Calorim. 2003;74:251–7.
Jona E, Horvath I, Kubranova M, Jorik V. Thermal decomposition reactions of nickel(II) complexes under quasi-equilibrium conditions. II. Study of the relations between thermal, spectral and diffraction properties of the Werner clathrates [Ni(4–Mepy)4-(NCS)2]·G, (G=benzene, toluene, p-xylene). J Therm Anal Calorim. 1993;41:187–96.
Czakis-Sulikowska D, Czylkowska A. Complexes of Mn(II), Co(II), Ni(II) and Cu(II) with 4, 4′-bipyridine and dichloroacetates. J Therm Anal Calorim. 2003;71:395–405.
Verma RK, Verma L, Ranjan M, Verma BP, Mojumdar SC. Thermal analysis of 2-oxocyclopentanedithiocarboxylato complexes of iron(III), copper(II) and zinc(II) containing pyridine or morpholine as the second ligand. J Therm Anal Calorim. 2008;94:27–31.
Madhurambal G, Ramasamy P, Anbusrinivasan P, Vasudevan G, Kavitha S, Mojumdar SC. Growth and characterization studies of 2-bromo-4′-chloro-acetophenone (BCAP) crystals. J Therm Anal Calorim. 2008;94:59–62.
Ukraintseva EA, Logvinenko VA, Soldatov DV, Chingina TA. Thermal dissociation processes for clathrates [CuPy4(NO3)2]·2G (G = tetrahydrofurane, chloroform). J Therm Anal Calorim. 2004;75:337–45.
Mojumdar SC, Melnik M, Jona E. Thermoanalytical investigation of magnesium(II) complexes with pyridine as bio-active ligand. J Therm Anal Calorim. 1999;56:541–6.
Rathore HS, Varshney G, Mojumdar SC, Saleh MT. Synthesis, characterization and fungicidal activity of zinc diethyldithiocarbamate and phosphate. J Therm Anal Calorim. 2007;90:681–6.
Mojumdar SC, Madhurambal G, Saleh MT. A study on synthesis and thermal, spectral and biological properties of carboxylato-Mg(II) and carboxylate-Cu(II) complexes with bioactive ligands. J Therm Anal Calorim. 2005;81:205–10.
Varshney KG, Agrawal A, Mojumdar SC. Pectin based cerium(IV) and thorium(IV) phosphates as novel hybrid fibrous ion exchangers synthesis, characterization and thermal behaviour. J Therm Anal Calorim. 2005;81:183–9.
Jona E, Rudinska E, Sapietova M, Pajtasova M, Ondrusova D, Jorik V, et al. Interaction of pyridine derivatives into the interlayer spaces of Cu(II)-montmorillonites. Res J Chem Environ. 2005;9:41–3.
Mojumdar SC, Miklovic J, Krutosikova A, Valigura D, Stewart JM. Furopyridines and furopyridine-Ni(II) complexes—synthesis, thermal and spectral characterization. J Therm Anal Calorim. 2005;81:211–5.
Mojumdar SC. Thermal properties, environmental deterioration and applications of macro-defect-free cements. Res J Chem Environ. 2005;9:23–7.
Madhurambal G, Mojumdar SC, Hariharan S, Ramasamy P. TG, DTC, FT-IR and Raman spectral analysis of Zna/Mgb ammonium sulfate mixed crystals. J Therm Anal Calorim. 2004;78:125–33.
Mojumdar SC. Thermoanalytical and IR-spectral investigation of Mg(II) complexes with heterocyclic ligands. J Therm Anal Calorim. 2001;64:629–36.
Kuznetsov VA, Okhrimenko TM, Rak M. Growth promoting effect of organic impurities on growth kinetics of KAP and KDP crystals. J Cryst Growth. 1998;193:164–73.
Nyvlt J, Rychly R, Gottfried J, Wurzelova J. Metastable zone-width of some aqueous solutions. J Cryst Growth. 1970;6:151–62.
Zaitseva NP, Rashkovich LN, Bogatyreva SV. Stability of KH2PO4 and K(H, D)2PO4 solutions at fast crystal growth rates. J Cryst Growth. 1995;148:276–82.
Buckley HE, editor. Crystal growth. New York: Wiley; 1951.
Silverstein R, Basseler GC, Morrill TC. Spectroscopic identification of organic compounds. 5th ed. New York: Wiley; 1998.
Rak M, Eremin NN, Eremina TA, Kuznetsov VA, Okhrimenko TM, Furmanova NG, et al. On the mechanism of impurity influence on growth kinetics and surface morphology of KDP crystals-I: defect centers formed by bivalent and trivalent impurity ions incorporated in KDP structure-theoretical study. J Cryst Growth. 2005;273:577–85.
Eremina TA, Kuznetsov VA, Eremin NN, Okhrimenko TM, Furmanova NG, Efremova EP, et al. On the mechanism of impurity influence on growth kinetics and surface morphology of KDP crystals—II: experimental study of influence of bivalent and trivalent impurity ions on growth kinetics and surface morphology of KDP crystals. J Cryst Growth. 2005;273:586–93.
Sangwal K. Effects of impurities on crystal growth processes. Prog Cryst Growth Charact Mater. 1996;32:3–43.
Lal K, Bhagavannarayan G. A high-resolution diffuse X-ray scattering study of defects in dislocation-free silicon crystals growth by the float-zone method and comparison with Czochralski-grown crystals. J Appl Cryst. 1989;22:209–15.
Ushasree PM, Jayavel R, Ramasamy P. Growth and characterization of phosphate mixed ZTS single crystals. Mater Sci Eng B. 1999;65:153–8.
Bhagavannarayana G, Choubey A, Shubin YV, Lal K. Study of point defects in as-grown and annealed bismuth germanate single crystals. J Appl Cryst. 2005;38:448–54.
Kurtz SK, Perry TT. A powder technique for the evaluation of nonlinear optical materials. J Appl Phys. 1968;39:3798–813.
Hall SR, Kolinsky PV, Jones R, Allen S, Gordon P, Bothwell B, et al. Polymorphism and nonlinear optical activity in organic crystals. J Cryst Growth. 1986;79:745–51.
Wang Y, Eaton DF. Optically non-linear organic molecules cyclodextrin inclusion complexes. Chem Phys Lett. 1985;120:441–4.
Acknowledgements
We like to thank the Annamalai University and National Physical Laboratory for all the facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meenakshisundaram, S.P., Parthiban, S., Kalavathy, R. et al. Thermal and optical properties of ZTS single crystals in the presence of 1,10-phenanthroline (Phen). J Therm Anal Calorim 100, 831–837 (2010). https://doi.org/10.1007/s10973-010-0762-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0762-4