Abstract
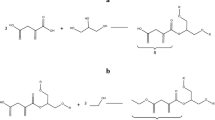
The title terpolymer (8-HQ5-SAMF-II) is synthesized by the condensation of 8-hydroxyquinoline 5-sulfonic acid (8-HQ5-SA) and melamine (M) with formaldehyde (F) in the presence of acid catalyst and using 2:1:3 M proportions of the reacting monomers. The synthesized terpolymer resin is then characterized by different physicochemical techniques viz. number average molecular mass determination, intrinsic viscosity determination, and spectral studies like UV–Visible, IR, 1H NMR, and 13C NMR spectra. The morphology of synthesized terpolymer was studied by scanning electron microscopy (SEM). The thermogravimetry of the terpolymer resin prepared in this study has been carried out by non-isothermal thermogravimetry technique in which sample is subjected to condition of continuous increase in temperature at linear rate. Thermal study of the resin was carried out to determine their mode of decomposition and relative thermal stabilities. Thermal decomposition curves were studied carefully with minute details. The Freeman-Carroll and Sharp-Wentworth methods have been used in the present investigation to calculate thermal activation energy and different kinetic parameter of the terpolymer resins. Thermal activation energy E a calculated with the two above-mentioned methods are in close agreement. The advantage of Freeman-Carroll method is to calculate both the order of reaction n and energy of activation in one single stage by keeping heating rate constant. By using data of thermogravimetry, various thermodynamic parameters like frequency factor Z, entropy change ΔS, free energy change ΔF, and apparent entropy S* have been determined using Freeman-Carroll method.









Similar content being viewed by others
References
Diaz FR, Moreno J, Tagle LH, East GA, Radic D. Synthesis, characterization and electrical properties of polyimines derived from selenophene. Synth Met. 1999;100:187–93.
Suh SC, Shim SC. Synthesis and properties of a novel polyazomethines, the polymer with high photoconductivity and second-order optical nonlinearity. Synth Met. 2000;114:91–5.
Bredas JL, Chance RR. Conjugated polymeric materials: opportunities in electronics optoelectronics and molecular electronics. Dordrecht: Kluwer Academic; 1990.
Marder SR, Sohn JR, Stucky GD. Materials for non-linear optics: chemical perspectives. Washington: ACS; 1991.
Ledoux-Rak I, Dodabalapur A, Blom P. Novel organic materials and technological advances for photonics. Synth Met. 2002;127:1–2.
Al Shawabkeh AF, Al Wahab HA, Shahab YA. Temperature dependence of the electrical conductivity of some conjugated polyazomethines. J Optoelectron Adv Mater. 2007;9:2075–7.
Nishide H, Yoshioka N, Tsuchida E, Inoue H. Coordination, structure and magnetic properties of poly(pyridilenemethylidenenitriloiron)s. J Polym Sci A. 1989;27:497–505.
Mazur M, Kaminska AM, Bukowska J. Surface catalyzed growth of poly (2-methoxyaniline) on gold. Electrochim Acta. 2007;52:5669–76.
Gupta RK, Singh RA. Solid-state organic batteries based on polymer composites of charge-transfer materials. J Polym Res. 2005;12:189–95.
Koval’chuk EP, Stratan NV, Reshetnyak OV, Blazejowski J, Wittingham MS. Synthesis and properties of the polyanisidines. Solid State Ionics. 2001;141:217–24.
Khuhawar MY, Shah A, Mughal MA. Preparation and characterization of Schiff base polymers derived from 4, 4′-methylenebis (cinnamaldehyde). J Polym Sci. 2007;25:399–407.
Cianga I, Ivanoiu M. Synthesis of poly(Schiff base)s by organometallic processes. Eur Polym J. 2006;42:1922–33.
Racles C, Cozen V, Sajo I. Influence of chemical structure on processing and thermo tropic properties of poly(siloxane-azomethine)s. High Perform Polym. 2007;19:541–52.
Bereket G, Hur E, Sahin Y. Electrochemical synthesis and anti-corrosive properties of polyaniline poly(2-anisidine) and poly(aniline-co-2-anisidine) films on stainless steel. Prog Org Coat. 2005;54:63–72.
Kaya I, Demir HO, Vilayetoglu AR. The synthesis and characterization of planar oligophenols with Schiff base substitute. Synth Met. 2002;126:183–91.
Kaya I, Koyuncu S. The synthesis and characterization of oligo-N-4-aminopyridine, oligo-2-[(pyridine-4-yl-imino) methyl] phenol and its some oligomer-metal complexes. Polymer. 2003;44:7299–309.
Guan LL, Zhang CX. Thermal stabilities and the thermal degradation kinetics of polyimide. Polym Degrad Stab. 2004;84:369–73.
Imai Y, Itoya K, Kakimoto M. Synthesis of aromatic polybenzoxazoles by silylation method and their thermal and mechanical properties. Macromol Chem Phys. 2000;201:2251–6.
Rahangdale PK, Gurnule WB, Paliwal LJ, Kharat RB. Synthesis of 4-hydroxyacetophenone-oxamide-formaldehyde terpolymer resins. In: Proceeding of national conference on recent trends in nanoscience; 2006. p. 222.
Tarase MV, Zade AB, Gurnule WB. Resin I. Synthesis, characterization and ion-exchange properties of terpolymer resins derived from 2,4-dihydreoxypropiophenone, biuret and formaldehyde. J Appl Polym Sci. 2008;108(2):738–46.
Pal TK, Kharat RB. Synthesis and characterization of salicylic acid-dithiobiuret-trioxane resins. J Indian Chem Soc. 1989;66:283–6.
Gurnule WB, Juneja HD, Paliwal LJ. Synthesis and thermogravimetric analysis of tercopolymers resin derived from salicylic acid, melamine and formaldehyde. J Indian Chem Sci. 1999;16:6–8.
Singru RN, Zade AB, Gurnule WB. Synthesis, characterization and thermal degradation studies of terpolymer resins derived from p-cresol, melamine and formaldehyde. J Appl Polym Sci. 2008;109(2):859–68.
Jadhao MM, Paliwal LJ, Bhave NS. Resin. III. Synthesis, characterization, and ion-exchange properties of a 2, 2′-dihydroxybiphenyl–formaldehyde. J Appl Polym Sci. 2008;109:508–14.
Morrison RT, Boyd RN. Organic chemistry. New Delhi: Prentice Hall of India Pvt. Ltd; 1996.
Barth Howard G, Mays Jimmy W. Modern methods of polymer characterization. New York: Willey; 1991.
Gurnule WB, Paliwal LJ, Kharat RB. Synthesis and stereo chemical characterization of polychelates derived from b, b′-(2-hydroxy-5-chlorobenzoyl)-p-divinylbenzene. Synth React Inorg Met Org Chem. 2001;31(8):1453–77.
Gupta RH, Zade AB, Gurnule WB. Thermal analysis studies of terpolymer resins derived from 2-hydroxyacetophenone, melamine and formaldehyde. J Ultra Sci Phys Sci. 2007;19(3):359–66.
Gurnule WB, Juneja HD, Paliwal LJ. Thermogravimetric analysis of p-hydroxybenzoic acid–melamine–formaldehyde tercopolymers. Asian J Chem. 1999;11:767–73.
Gurnule WB, Juneja HD, Paliwal LJ. Thermal degradation of 8-hydroxyquinonline–biuret–formaldehyde tercopolymers. In: Proceeding of international congress of chemistry and environment, Indore; 2001. p. 98–101.
Katkamwar SS, Zade AB, Gurnule WB. Thermogravimetric analysis of terpolymer resins derived from 8-hydroxyquinoline-dithiooxamide and formaldehyde. J Ultra Sci Phys Sci. 2007;3(2):103–8.
Tarase MV, Zade AB, Gurnule WB. Thermal degradation studies of terpolymer resins derived from 2,4-dihydreoxypropiophenone, biuret and formaldehyde. J Ultra Chem. 2007;3(1):41–8.
Tamargo MK, Villar RS, Paredes JI. Studies on the thermal degradation of poly (p-phenylene benzobisoxazole). Chem Mater. 2003;15:4052–9.
Nielsen CA, Pierini P, Fuh S. Thermal and thermo oxidative degradation of poly (p-phenylene-cis-benzobisoxazole) (PBO): determination of kinetics and reaction products. J Fire Sci. 1993;11:156–71.
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogel’s text book of practical organic chemistry. England: Addison Wesley Longman Ltd; 1998.
Acknowledgements
The authors are grateful to Director and Head, Department of Chemistry, L.I.T., R.T.M. Nagpur University, Nagpur, for providing laboratory facility. They are also thankful to the Director, SAIF, Punjab University, Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singru, R.N., Gurnule, W.B. Thermogravimetric study of 8-hydroxyquinoline 5-sulfonic acid–melamine–formaldehyde terpolymer resins-II. J Therm Anal Calorim 100, 1027–1036 (2010). https://doi.org/10.1007/s10973-010-0672-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0672-5