Abstract
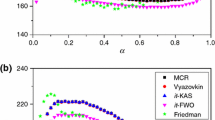
Dependence of kinetic parameters (activation energy and pre-exponential factor) and procedural factors (sample mass and heating rate) independent of the reversibility and the type of reactions in non-isothermal thermogravimetry have been established. Tris(ethylenediamine)nickel(II) oxalate dihydrate has been selected as a model complex and experiments were carried out at different heating rates and sample masses to study the dependence quantitatively. The kinetic parameters calculated using mechanistic and non-mechanistic equations show a systematic decrease with increase in either sample mass or heating rate for the dehydration and deamination reactions. For the decomposition reaction, the kinetic parameters are not influenced by the procedural factors. Mathematical correlations of high reliability are established between kinetic parameters and heating rate/sample mass using both mechanistic and non-mechanistic equations for dehydration and deamination reactions. The quantification follows an exponential decay of second order relation with respect to heating rate and a sigmoidal relation with regard to sample mass for both the dehydration and deamination reactions. No quantitative correlation is possible for the final decomposition stage. Thus, it is found that independent of the type of reaction (deamination or dehydration) the kinetic parameters have a particular dependence on the procedural variables. The equations for exponential decay and sigmoidal dependence can be represented as \( y = y_{0} + A_{1} {\text{e}}^{{ - x/t_{1} }} + A_{2} {\text{e}}^{{ - x/t_{2} }} \) and \( y = {\frac{{A_{1} - A_{2} }}{{1 + {\text{e}}^{{(x - x_{0} )/{\text{d}}x}} }}} + A_{2} \) respectively, where y represents kinetic parameters (E or A) and x represents the procedural variables (φ or m). Mechanism of the dehydration reaction is found to be random nucleation with the formation of one nucleus on each particle and the deamination is a phase boundary reaction. It is observed that the mechanism of these reversible reactions is not affected by the variation in sample mass and heating rate.





Similar content being viewed by others
References
Krishnan K, Ninan KN, Madhusudanan PM. Effect of simultaneous variation of sample mass and heating rate on the mechanism of dehydration of zinc oxalate dihydrate. Thermochim Acta. 1985;89:295–305.
Ubaldini A, Artini C, Costa GA, Carnasciali MM, Masini R. Synthesis and thermal decomposition of mixed Gd–Nd oxalates. J Therm Anal Calorim. 2008;91:797–803.
Su TT, Zhai YC, Jiang H, Gong H. Studies on the thermal decomposition kinetics and mechanism of ammonium niobium oxalate. J Therm Anal Calorim. 2009;98:449–455.
Thomas P, Dwarakanath K, Varma KBR, Kutty TRN. Synthesis of nanoparticles of the giant dielectric material, CaCu3Ti4O12 from a precursor route. J Therm Anal Calorim. 2009;95:267–72.
Rejitha KS, Mathew S. Thermal deamination kinetics of tris(ethylenediamine)nickel(II) sulphate in the solid-state. J Therm Anal Calorim. 2008;93:213–7.
Mathew S, Nair CGR, Ninan KN. Quantitative correlations of activation parameters and procedural factors-dependence on reaction type. Thermochim Acta. 1991;184:269–94.
Shishkin YL. The effect of sample mass and heating rate on DSC results when studying the fractional composition and oxidative stability of lube base oils. Thermochim Acta. 2006;444:26–34.
Bigda R, Mianowsky A. Influence of heating rate on kinetic quantities of solid phase thermal decomposition. J Them Anal Calorim. 2006;84:453–65.
Olszak-Humienik M, Możejko J. Eyring parameters of dehydration processes. Thermochim Acta. 2003;405:171–81.
Liu Y, Zhao J, Zhang H, Zhu Y, Wang Z. Thermal decomposition of basic zinc carbonate in nitrogen atmosphere. Thermochim Acta. 2004;414:121–3.
Samtani M, Dollimore D, Alexander KS. Comparison of dolomite decomposition kinetics with related carbonates and the effect of procedural variables on its kinetic parameters. Thermochim Acta. 2002;392–393:135–45.
Haschke JM, Wendlandt WW. The thermal decomposition of metal ethylenediamine complexes. Anal Chim Acta. 1965;32:386–93.
Vogel AG. Text book of quantitative inorganic analysis. 4th ed. London: Longmann; 1978.
Maciejewski M. Somewhere between fiction and reality—the usefulness of kinetic data of solid-state reactions. J Therm Anal. 1992;38:51–70.
Wendlandt WW. Thermal methods of analysis. 3rd ed. New York: Wiley-Interscience; 1986.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
MacCallum JR, Tanner J. The kinetics of thermogravimetry. Eur Polym J. 1970;6:1033–9.
Horowitz HH, Metzger G. A new analysis of thermo gravimetric traces. Anal Chem. 1963;35:1464–8.
Madhusudanan PM, Krishnan K, Ninan KN. New approximation for the p(x) function in the evaluation of non-isothermal kinetic data. Thermochim Acta. 1986;97:189–201.
Spiegel MR. Theory and problems of statistics in SI units. 1st ed. New York: McGraw-Hill; 1972.
Freud JE. Mathematical statistics. 5th ed. New Jersey: Prentice Hall Inc; 1992.
Satava V. Mechanism and kinetics from non-isothermal TG traces. Thermochim Acta. 1971;2:423–8.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rejitha, K.S., Mathew, S. Quantitative correlation of kinetic parameters and procedural factors in non-isothermal thermogravimetry-dependence of heating rate and sample mass. J Therm Anal Calorim 100, 909–916 (2010). https://doi.org/10.1007/s10973-009-0670-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0670-7