Abstract
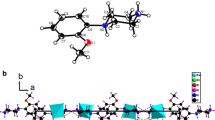
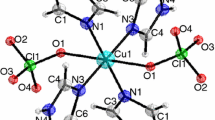
The complex (C11H18NO)2CuCl4(s) was synthesized. Chemical analysis, elemental analysis, and X-ray crystallography were used to characterize the structure and composition of the complex. Low-temperature heat-capacities of the compound were measured by an adiabatic calorimeter in the temperature range from 77 to 400 K. A phase transition of the compound took place in the region of 297–368 K. Experimental molar heat-capacities were fitted to two polynomial equations of heat-capacities as a function of the reduced temperature by least square method. The peak temperature, molar enthalpy, and entropy of phase transition of the compound were calculated to be T trs = 354.214 ± 0.298 K, Δtrs H m = 76.327 ± 0.328 kJ mol−1, and Δtrs S m = 51.340 ± 0.164 J K−1 mol−1.




Similar content being viewed by others
References
Chinese Pharmacopoeia Committee of Health Ministry of PR China. Chinese Pharmacopoeia. Vol. II. Beijing: Chemical Industry Press; 1990. p. 528.
Di YY, Gao WJ, Yang WW, Kong YX, Tan ZC. Synthesis, characterization, and thermodynamic study of the coordination compound Cd(HNic)2Cl2(s). J Chem Eng Data. 2008;53:1602–6.
Tan ZC, Shi Q, Liu BP, Zhang HT. A fully automated adiabatic calorimeter for heat capacity measurement between 80 and 400 K. J Therm Anal Calorim. 2008;92:367–74.
Lan XZ, Tan ZC, Shi Q, Gao ZH. Gelled Na2HPO4···12H2O with amylose-g-sodium acrylate: heat storage performance, heat capacity and heat of fusion. J Therm Anal Calorim. 2009;96:1035–40.
Yin HD, Chen SW, Li LW, Wang DQ. Synthesis, characterization and crystal structures of the organotin(IV) compounds with the Schiff base ligands of pyruvic acid thiophene-2-carboxylic hydrazone and salicylaldehyde thiophene-2-carboxylic hydrazone. Inorg Chim Acta. 2007;360:2215–23.
Di YY, Tan ZC, Li LW, Gao SL, Sun LX. Low-temperature heat capacities and standard molar enthalpy of formation of the complex Zn(Val)SO4·H2O(s) (Val = L-α-valine). J Therm Anal Calorim. 2007;89:545–51.
Ditmars DA, Ishihara S, Chang SS, Bernstein G, West ED. Enthalpy and heat-capacity standard reference material: synthetic sapphire (α-Al2O3) from 10 to 2250 K. J Res Natl Bur Stand. 1982;87:159–63.
Xu RY, Kong DJ, Cai XE, Zhu J. Studies of solid-solid phase transitions for (n-C18H37NH3)2MCl4. Thermochim Acta. 1990;164:307–14.
Tan ZC, Sun LX, Meng SH, Li L, Xu F, Yu P. Heat capacities and thermodynamic functions of p-chlorobenzoic acid. J Chem Thermodyn. 2002;34:1417–29.
Di YY, Tan ZC X, Wu M, Meng SH, Qu SS. Heat capacity and thermochemical study of trifloroacetamide (C2H2F3NO). Thermochim Acta. 2000;356:143–51.
Acknowledgements
This work was financially supported by the National Natural Science Foundations of China under the contract NSFC No. 20673050 and 20973089.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dan, W.Y., Di, Y.Y., Kong, Y.X. et al. Crystal structure and solid–solid phase transition of the complex (C11H18NO)2CuCl4(s). J Therm Anal Calorim 102, 291–296 (2010). https://doi.org/10.1007/s10973-009-0663-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0663-6