Abstract
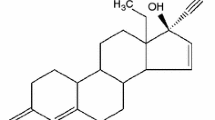
Tibolone polymorphic forms I (monoclinic) and II (triclinic) have been prepared by recrystallization from acetone and toluene, respectively, and characterized by different techniques sensitive to changes in solid state, such as polarized light microscopy, X-ray powder diffractometry, thermal analysis (TG/DTG/DSC), and vibrational spectroscopy (FTIR and Raman microscopy). The nonisothermal decomposition kinetics of the obtained polymorphs were studied using thermogravimetry. The activation energies were calculated through the Ozawa’s method for the first step of decomposition, the triclinic form showed a lower E a (91 kJ mol−1) than the monoclinic one (95 kJ mol−1). Furthermore, Raman microscopy and DSC at low heating rates were used to identify and follow the thermal decomposition of the triclinic form, showing the existence of three thermal events before the first mass loss.






Similar content being viewed by others
References
Rymer J. Why tibolone is different? Rev Gynaecol Pract. 2002;2:165–70.
Organon NV. 7-Methylestrenes. 6 pp; 1965. NL 6406797 19651217 Application: NL 19640615. Priority: NL 19640615. CAN 64:68087 AN 1966:68087.
Wieland P, Anner G. Steroids. Synthesis of 7alpha-methyl-3-oxo-19-norandrosta-4, 9, 11-trienes. Helv Chim Acta. 1967;50(6):1453–61.
Schouten A, Kanters JA. Structure of the triclinic modification of 17β-hydroxy-19-nor-7α-methyl-17α-pregn-5(10)-en-20-in-3-one (Org OD 14). Acta Crystallogr C. 1991;47:1754–6.
Declercq JP, Van Meerssche M, Zeelen FJ. Conformational analysis of 3-oxo 5(10)-unsaturated steroids. Single-crystal X-ray structure analysis of 17-hydroxy-7α-7-pregn-5(10)-en-20-in-3-one (Org OD 14). Recl Trav Chim Pays Bas. 1984;103:145–7.
Sas GAJMT, Van Doornum EM. Pharmaceutical composition which contains a pharmaceutically suitable carrier and the compound having the structure (7 alpha, 17 alpha)-17-hydroxy-7-methyl-19-nor-17-pregn-5(10)-en-20-yn-3-one. EP Pat. 0 389 035; 1990.
Boerrigter SXM, Van Den Hoogenhof CJM, Meekes H, Bennema P, Vlieg E. In situ observation of epitaxial polymorphic nucleation of the model steroid methyl analogue 17 norethindrone. J Phys Chem B. 2002;106:4725–31.
Booy K, Wiegerinck P, Vader J, Kaspersen F, Lambregts D, Vromans H, et al. The use of 13C labeling to enhance the sensitivity of 13C solid-state CPMAS NMR to study polymorphism in low dose solid formulations. J Pharm Sci. 2005;94(2):458–63.
Ozawa T. A new method of analyzing thermogravimetric data. J Bull Chem Soc Jpn. 1965;38(11):1881–6.
Felix FS, Cides da Silva LC, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95(3):877–80.
Budavari S, editor. The Merck index. 11th ed. N.J.: Rahway; 1989. p. 1485.
Stoica C, Tinnemans P, Meekes H, Vlieg E. Epitaxial 2D nucleation of metastable polymorphs: a 2D version of Ostwald’s rule of stages. Cryst Growth Des. 2005;5(3):975–81.
Stoica C, Verwer P, Meekes H, Vlieg E, Van Hoof PJCM, Kaspersen FM. Epitaxial 2D nucleation of the stable polymorphic form of the steroid 7MNa on the metastable form: Implications for Ostwald’s rule of stages. Int J Pharm. 2006;309(1–2):16–24.
Acknowledgements
The authors would like to thank Professor José Moacyr Vianna Coutinho for his kind help with the microscopy experiments and Sintefina Indústria e Comércio LTDA for kindly providing the starting material. The authors acknowledge also Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Araujo, G.L.B., de Faria, D.L.A., Zaim, M.H. et al. Thermal studies on polymorphic structures of tibolone. J Therm Anal Calorim 102, 233–241 (2010). https://doi.org/10.1007/s10973-009-0580-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0580-8