Abstract
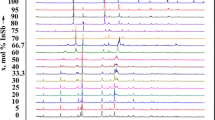
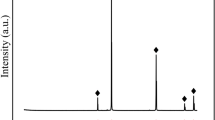
The study of the system xSb2O3–(1 − x)Bi2O3–6(NH4)2HPO4 has been carried out to identify the phases and simulate the mechanisms of their formation, using the technique of thermal analysis in association with X-ray diffractometry. The main stages observed during thermal treatment of the samples include: (1) elimination of water and ammonia leading to the formation of (NH4)5P3O10; (2) reaction of the latter with M III2 O3 and the formation of acidic polyphosphates M III2 H2P3O10; (3) their dehydration with the formation of the polyphosphates MIII(PO3)3. Then Sb(PO3)3 decomposes giving SbPO4 and P2O5. In the presence of excessive P2O5, two moles of Bi(PO3)3 condensate into oxophosphates Bi2P4O13 and BiP5O14. The data of thermal analysis match with the composition of intermediate and final products. The hygroscopicity of the samples diminishes with growing bismuth content.





Similar content being viewed by others
References
Kinberger B. Crystal structure of antimony phosphate. Acta Chem Scand. 1970;214:320–8.
Kurbanov KM. On crystal structure of antimony phosphate SbPO4. Kristallografia. 1987;32:1265–7.
Melnikov P, Secco MAC, Guimarães WR, dos Santos HWL. Thermochemistry of vitreous antimony orthophosphate. J Therm Anal Calorim. 2008;92:579–82.
Romero B, Bruque S, Aranda MAG, Iglesias JE. Syntheses, crystal structures, and characterization of bismuth phosphates. Inorg Chem. 1994;33:1869–74.
Ni Yu, Hughes JM, Mariano AN. Crystal-chemistry of the monazite and xenotime structures. Am Mineral. 1995;80:21–6.
Saadouri H, Boukhari A, Flandrois S, Aristide J. Intercalation of hydrazine and amines in antimony phosphate. Mol Cryst Liq Cryst. 1994;244:173–6.
Alonzo G, Bertazzi N, Galli P, Marci G, Massucci MA, Palmisano L, et al. In search of layered antimony(III) materials: synthesis and characterization of oxo-antimony(III) catecholate and further studies on antimony(III) phosphate. Mater Res Bull. 1998;33:1233–40.
Chang TS, Li GJ, Shin CH, Lee YK, Yun SS. Catalytic behavior of BiPO4 in the multicomponent bismuth phosphate system on the propylene ammoxidation. Catal Lett. 2000;68:229–34.
Takita Y, Ninomiya M, Miyake H, Wakamatsu H, Yoshinaga Y. Catalytic decomposition of perfluorocarbons: Part II—decomposition of CF4 over AlPO4-rare earth phosphate catalysts. Phys Chem Chem Phys. 1999;1:4501–4.
Iitaka K, Tani Y, Umezawa Y. Orthophosphate ion-sensors based on a quartz-crystal microbalance coated with insoluble orthophosphate salts. Anal Chim Acta. 1997;338:77–87.
Charyulu MM, Chetty KV, Phal DG, Sagar V, Naronha DM, Pawar SM, et al. Recovery of americium from nitric acid solutions containing calcium by different co-precipitation methods. J Rad Nucl Chem. 2002;251:153–4.
Jermoumi T, Hafid M, Et-tabirou M, Taibi M, ElQadim H, Toreis N. Electrical conductivity study on Na3PO4–Pb3(PO4)2–BiPO4. Mater Sci Eng B. 2001;85:28–33.
Melnikov P, dos Santos FJ, Santagnelli SB, Secco MAC, Guimarães WR, Delben A, et al. Mechanism of the formation and properties of antimony polyphosphate. J Therm Anal Calorim. 2005;81:45–9.
Kulaev IS, Vagabov VM, Kulakovskaya TV. The structure of condensed phosphates. In: Kulaev IS, Vagabov VM, Kulakovskaya TV, editors. The biochemistry of inorganic polyphosphates. New York: Wiley; 2004.
Van Wezer JR. Phosphorus and its compounds, vol. 1. New York: Interscience Publishers; 1958.
Melnikov P, Guirardi AL, Secco MAC, de Aguiar EN. Study of trivalent elements polyphosphates by thermal analysis. J Therm Anal Calorim. 2008;94:162–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melnikov, P., dos Santos, H.W.L. & Gonçalves, R.V. Thermal behavior of the mixed composition xSb2O3–(1 − x)Bi2O3–6(NH4)2HPO4 . J Therm Anal Calorim 101, 907–911 (2010). https://doi.org/10.1007/s10973-009-0551-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0551-0