Abstract
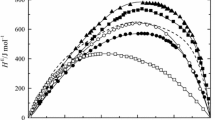
Enthalpies of mixing of ethanol solution of R- and S-enantiomers of limonene in large concentration have been measured at 298.15 K. The enthalpies of mixing were negligibly small for all concentrations. Enthalpies of mixing showed negative in less than 30 mol%, but positive in more than the high concentration of limonenes. The heterochiral solutions were more stable than each of the homochiral solutions in dilute solutions. The concentration dependence on enthalpies of mixing in dilute concentration of less than 10 mol% was much sharper in inclination than the dense solutions limonene.




Similar content being viewed by others
References
Takagi S, Fujishiro R, Amaya K. Heats of mixing optical isomers in solution: calorimetric evidence of the stereospecific effect. J Chem Soc Chem Commun. 1968;1968:480.
Guette JP, Boucherot D, Horeau A. Diastereoisomeric interactions of enantiomers in the liquid phase. II. Can one separate the antipodes of a chiral compound by distillation. Tetrahedron Lett. 1973;1973:465–8.
Atik Z, Ewing MB, McGlashan ML. Chiral discrimination in liquids. Excess molar volumes of (1−x)A+ + xA−, where A denotes limonene, fenchone, and α-methylbenzylamine. J Phys Chem. 1981;85:3300–3.
Atik Z, Ewing MB, McGlashan ML. Chiral discrimination in liquids. II. Excess molar enthalpies of {(1−x)A+ + xA−}, where A denotes fenchone or α-methylbenzylamine. J Chem Thermodyn. 1983;15:159–63.
Lepori L, Koppenhoefer B. Chiral discrimination in the liquid phase: excess volumes of binary mixtures of amino acid derivatives. J Phys Chem. 1994;98:6862–4.
Kimura T, Ozaki T, Takagi S. Deuterium isotope effect on excess enthalpies of methanol or ethanol and their deuterium derivatives. Chirality. 1998;10:5–275.
Kimura T, Ozaki T, Takagi S. Enthalpy changes observed upon mixing liquid (R)- and (S)-enantiomers at 298.15 K. Enantiomers. 2001;6:5–17.
Kimura T, Matsushita T, Ueda K, Matsuda T, Aktar F, Kamiyama T, et al. Enthalpy changes on mixing two couples of S- and R-enantiomers of heptane-2-ol, octane-2-ol, nonane-2-ol, 3-chloro-propane-1, 2-diol, 2-methyl-1, 4-butanediol at 298.15 K. Thermochim Acta. 2004;414:209–14.
Kimura T, Khan MA, Ishii M, Ueda K, Matsushita T, Kamiyama T, et al. Enthalpic changes on mixing two couples of S- and R-enantiomers of benzyl-(1-phenyl–ethyl)-amine, 1-phenylethylamine, 1-phenyl-ethanol, butyric acid oxiranylmethyl ester, 4-methyl-[1, 3]dioxolan-2-one, 2-Chloro- methyloxirane at T = 298.15 K. J Chem Thermodyn. 2006;38:1042–8.
Kimura T, Khan MA, Kamiyama T. Enthalpy change on mixing a couple of some chiral compounds at 298.15 K. Chirality. 2006;18:581–6.
Kimura T, Khan MA, Kamiyama T, Fujisawa M. Thermodynamic properties of d- and l-tartaric acid in aqueous and ethanol solution at 298.15 K. J Chem Eng Data. 2006;51:909–13.
Kimura T, Khan MA, Kamiyama T. Enthalpic changes on mixing two couples of S- and R-enantiomers which contained amino groups at 298.15 K. J Therm Anal Calorim. 2006;85:575–80.
Kimura T, Iwama S, Kido S, Abdullah Khan M. Enthalpies of mixing of ethanol solution of chiral camphor and its derivatives. J Chem Thermodyn. 2009;41:1170–7.
Kimura T, Ozaki T, Takeda S, Nakai Y, Takagi S. Excess enthalpies of binary mixtures of propanediamine + propanediol at 298.15 K. J Therm Anal Calorim. 1998;54:285–96.
Kimura T, Matsushita T, Ueda K, Tamura T, Takagi S. Deuterium isotope effect on excess enthalpies of methanol or ethanol and their deuterium derivatives. J Therm Anal Calorim. 2001;64:231–41.
Kimura T, Matsushita T, Ueda K, Kamiyama T, Takagi S. Excess enthalpies of {C n H2n+1CN, n = 4 ~ 12)} + methyl methylthiomethyl sulfoxide or + dimethyl sulfoxide at 298.15 K. J Chem Eng Data. 2004;49:1046–51.
Kimura T, Usui Y, Nishimura S, Takagi S. Measurement of excess volume of (Benzene+Cyclohexane) at 298.15 K as a reliability test for a vibration-tube density meter DMA 55. J Fac Sci Technol Kinki Univ. 1989;25:109–16.
Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh, PA; 2003.
Hildebrand JH, Prausnitz JM, Scott RL. Regular and related solutions. New York: Van Nostrand Reinhold Company; 1970.
James C, Acree WE Jr. Enthalpies of vaporization of organic and organometallic compounds 1880–2002. J Phys Chem Ref Data. 2003;32:519–87.
Shinoda K. Principles of solution and solubilities. New York: Marcel Dekker; 1978. p. 8–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, T., Kido, S. Enthalpies of mixing of ethanol solution of chiral limonenes at 298.15 K. J Therm Anal Calorim 99, 87–93 (2010). https://doi.org/10.1007/s10973-009-0482-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0482-9