Abstract
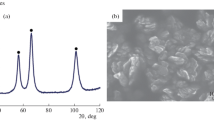
The metal complex, [Ni(en)2(H2O)2](NO3)2 (en = ethylenediamine), was decomposed in a static furnace at 200 °C by autogenous decomposition to obtain phase pure metallic nickel nanocrystallites. The nickel metal thus obtained was studied by XRD, IR spectra, SEM and CHN analysis. The nickel crystallites are in the nanometer range as indicated by XRD studies. The IR spectral studies and CHN analyses show that the surface is covered with a nitrogen containing species. Thermogravimetric mass gain shows that the product purity is high (93%). The formed nickel is stable and resistant to oxidation up to 350 °C probably due to the coverage of nitrogen containing species. Activation energy for the oxidation of the prepared nickel nanocrystallites was determined by non-isothermal methods and was found to depend on the conversion ratio. The oxidation kinetics of the nickel crystallites obeyed a Johnson–Mehl–Avrami mechanism probably due to the special morphology and crystallite strain present on the metal.










Similar content being viewed by others
References
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–6.
Kumar D, Zhou H, Nath TK, Kvit AV, Narayan J, Craciun V, et al. Improved magnetic properties of self-assembled epitaxial nickel nanocrystallites in thin-film ceramic matrix. J Mater Res. 2002;17:738–42.
Chu SZ, Wada K, Inoue S, Todoroki SI, Takahashi YK, Hono K. Fabrication and characteristics of ordered Ni nanostructures on glass by anodization and direct current electrodeposition. Chem Mater. 2002;14:4595–602.
Green M, O’Brien P. The preparation of organically functionalised chromium and nickel nanoparticles. Chem Commun 2001;1912–3.
Yu K, Katabi G, Cao X, Prozorov R, Gedanken A. Sonochemical preparation of amorphous nickel. J Non-Cryst Solids. 1996;201:159–62.
Degen A, Macek J. Preparation of submicrometer nickel powders by the reduction from nonaqueous media. Nano Struct Mater. 1999;12:225–8.
Davis SC, Klabunde KJ. Unsupported small metal particles: preparation, reactivity, and characterization. Chem Rev. 1982;82:153–208.
Ni X, Zhao Q, Zhang D, Yang D, Zheng H. Large scaled synthesis of chainlike nickel wires assisted by ligands. J Cryst Growth. 2005;280:217–21.
Wang H, Jiao X, Chen D. Monodispersed nickel nanoparticles with tunable phase and size: synthesis, characterization, and magnetic properties. J Phys Chem C. 2008;112:18793–7.
Park J, Kang E, Son SU, Park HM, Lee MK, Kim J, et al. Monodisperse nanoparticles of Ni and NiO: synthesis, characterization, self-assembled superlattices, and catalytic applications in the Suzuki coupling reaction. Adv Mater. 2005;17:429–34.
Rejitha KS, Mathew S. Thermal deamination kinetics of tris(ethylenediamine)nickel(II) sulphate in the solid-state. J Therm Anal Calorim. 2008;93:213–7.
Schimpf S, Louis C, Claus P. Ni/SiO2 catalysts prepared with ethylenediamine nickel precursors: influence of the pretreatment on the catalytic properties in glucose hydrogenation. Appl Catal A. 2007;318:45–53.
Negrier F, Marceau E, Che M, Giraudon JM, Gengembre L, Lofberg A. A systematic study of the interactions between chemical partners (metal, ligands, counterions, and support) involved in the design of Al2O3-supported nickel catalysts from diamine–Ni(II) chelates. J Phys Chem B. 2005;109:2836–45.
Atkinson A, Taylor RI. The diffusion of 63Ni along grain boundaries in nickel oxide. Philos Mag A. 1981;43:979–98.
Zhou L, Rai A, Piekiel N, Ma X, Zachariah MR. Ion-mobility spectrometry of nickel nanoparticle oxidation kinetics: application to energetic materials. J Phys Chem C. 2008;112:16209–18.
Song P, Wen D, Guo ZX, Korakianitis T. Oxidation investigation of nickel nanoparticles. Phys Chem Chem Phys. 2008;10:5057–65.
Suwanwatana W, Yarlagadda S, Gillespie JW. An investigation of oxidation effects on hysteresis heating of nickel particles. J Mater Sci. 2003;38:565–73.
Karmhag R, Tesfamichael T, Wackelgard E, Niklasson GA, Nygren M. Oxidation kinetics of nickel particles: comparison between free particles and particles in an oxide matrix. Sol Energy. 2000;68:329–33.
Negrier F, Marceau E, Che M, de Caro D. Role of ethylenediamine in the preparation of alumina-supported Ni catalysts from [Ni(en)2(H2O)2](NO3)2: from solution properties to nickel particles. C R Chimie. 2003;6:231–40.
Suryanarayana C, Norton MG. X-ray diffraction a practical approach. New York: Plenum Press; 1998.
Curtis NF, Curtis YM. Some nitrato-amine Nickel(II) compounds with monodentate and bidentate nitrate ions. Inorg Chem. 1965;4:804–9.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds part B: applications in coordination, organometallic, and bioinorganic chemistry. 5th ed. New York: Wiley; 1997.
Sun KQ, Marceau E, Che M. Evolution of nickel speciation during preparation of Ni–SiO2 catalysts: effect of the number of chelating ligands in [Ni(en)x(H2O)6-2x]2+ precursor complexes. Phys Chem Chem Phys. 2006;8:1731–8.
JCPDS card No: 04-0850.
Guerlou GL, Delmas C. Structure and properties of precipitated nickel-iron hydroxides. J Power Sour. 1993;45:281–9.
JCPDS card No: 47-1049.
Karmhag R, Niklasson GA, Nygren M. Oxidation kinetics of nickel nanoparticles. J Appl Phys. 2001;89:3012–7.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1996;4:323–8.
Akahira T, Sunose T. Research report of Chiba Institute Technology. 1971;16:22.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci B. 1969;7:41–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Gotor FJ, Criado JM, Malek J, Koga N. Kinetic analysis of solid-state reactions: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A. 2000;104:10777–82.
Courtade L, Turquat Ch, Muller Ch, Lisoni JG, Goux L, Wouters DJ, et al. Oxidation kinetics of Ni metallic films: formation of NiO-based resistive switching structures. Thin Solid Films. 2008;516:4083–92.
Ren YL, Wang X, Shui M, Li RS. The influence of morphology of ultra-fine calcite particles on decomposition kinetics. J Therm Anal Calorim. 2008;91:867–71.
Acknowledgements
The authors thank Department of Science and Technology, India for using the Sophisticated Analytical Instrument Facility (SAIF) at the Sophisticated Test and Instrumentation centre (STIC), Cochin University of Science and Technology, Cochin, for SEM analysis. S. Manju thanks Kerala State Council for Science, Technology and Environment for research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Robinson, P.P., Arun, V., Manju, S. et al. Oxidation kinetics of nickel nano crystallites obtained by controlled thermolysis of diaquabis(ethylenediamine)nickel(II) nitrate. J Therm Anal Calorim 100, 733–740 (2010). https://doi.org/10.1007/s10973-009-0209-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0209-y