Abstract
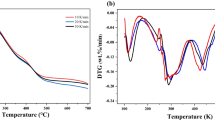
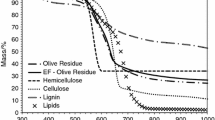
In pseudo bi-component separated-stage model (PBSM), the effect of the TG value at separation points on the kinetic parameters is studied by residual and theoretical analysis. Simultaneously, a new method to determine the point that is the end of 1st reaction or the initial of 2nd reaction is developed. The investigations have improved the calculation procedure of PBSM. We performed thermogravimetry (TG) analysis on oil tea wood with two-step consecutive model and parallel model. Comparison between the results of the two models and improved PBSM shows well agreements. The influence of different separation points on kinetic parameters is presented.












Similar content being viewed by others
References
Scuracchio CH, Waki DA, da Silva MLCP. Thermal analysis of ground tire rubber devulcanized by microwaves. J Therm Anal Calorim. 2007;87:893–7.
Valkova D, Kislinger J, Pekar M, Kucerik J. The kinetics of thermo-oxidative humic acids degradation studied by isoconversional methods. J Therm Anal Calorim. 2007;89:957–64.
Dweck J. Qualitative and quantitative characterization of Brazilian natural and organophilic clays by thermal analysis. J Therm Anal Calorim. 2008;92:129–35.
Thomas PS, Guerbois JP, Russel GH, Briscoe BJ. FTIR study of the thermal degradation of poly(vinyl alcohol). J Therm Anal Calorim. 2001;64:501–8.
Budrugeac P. Kinetics of the complex process of thermo-oxidative degradation of poly(vinyl alcohol). J Therm Anal Calorim. 2008;92:291–6.
Momoh M, Eboatu A. Thermogravimetric studies of the pyrolytic behaviour in air of selected tropical timbers. Fire Mater. 1996;20:173–81.
Blasi CDi, Branca C. Global degradation kinetics of wood and agricultural residues in air. Can J Chem Eng. 1999;77:555–61.
Meszaros E, Varhegyi G, Jakab E. Thermogravimetric and reaction kinetic analysis of biomass samples from an energy plantation. Energy Fuels. 2004;18:497–507.
Caballero JA, Font R, Maricilla A. Comparative study of the pyrolysis of almond shells and their fractions, holocellulose and lignin. Thermochim Acta. 1996;276:57–77.
Font R, Marcilla A, Verdu E, Devesa J. Thermogravimetric kinetic study of the pyrolysis of almond shells and almond shells impregnated with CoCl2. J Anal Appl Pyrol. 1991;21:249–64.
Di Blasi C, Lanzetta M. Intrinsic kinetics of xylan degradation in inert atmosphere. J Anal Appl Pyrol. 1997;40–41:287–303.
Di Blasi C, Signorelli G, Di Russo C, Rea G. Product distribution from pyrolysis of wood and agricultural residues. Ind Eng Chem Res. 1999;38:2216–24.
Varhegyi G, Antal MJ, Jakab E, Szabo P. Kinetic modeling of biomass pyrolysis. J Anal Appl Pyrol. 1997;42:73–87.
Manya JJ, Velo E, Puigjaner L. Kinetics of biomass pyrolysis: a reformulated three-parallel-reactions model. Ind Eng Chem Res. 2003;42:434–41.
Chen HX, Liu NA, Fan WC. Two-step consecutive reaction model and kinetic parameters relevant to the decomposition of Chinese forest fuels. J Appl Polym Sci. 2006;102:571–6.
Varhegyi G, Antal MJ, Szabo P, Jakab E, Hill F. Application of complex reaction kinetic models in thermal analysis. J Therm Anal Calorim. 1996;47:535–42.
Varhegyi G, Sazabo P, Jakab E, Till F. Least squares criteria for the kinetic evaluation of thermoanalytical experiments. Examples from a char reactivity study. J Anal Appl Pyrol. 2001;57:203–22.
Liu NA, Fan WC, Dobashi R. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J Anal Appl Pyrol. 2002;63:303–25.
Wendlandt WW. Thermal analysis. New York: Wiley; 1986.
Acknowledgements
This research was supported by National Natural Science Foundation of China (Grant No: 50536030) and Program for New Century Excellent Talents in University (NCET-05-0551). The authors deeply appreciate the supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, J., Yang, L., Zhou, Y. et al. Effect of separation points on kinetic parameters in pseudo component separated stage model. J Therm Anal Calorim 100, 599–605 (2010). https://doi.org/10.1007/s10973-009-0208-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0208-z