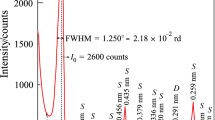
Abstract
Controlled rate thermal analysis (CRTA) technology offers better resolution and a more detailed interpretation of the decomposition processes of a clay mineral such as sepiolite via approaching equilibrium conditions of decomposition through the elimination of the slow transfer of heat to the sample as a controlling parameter on the process of decomposition. Constant-rate decomposition processes of non-isothermal nature reveal changes in the sepiolite as the sepiolite is converted to an anhydride. In the dynamic experiment two dehydration steps are observed over the ~20–170 and 170–350 °C temperature range. In the dynamic experiment three dehydroxylation steps are observed over the temperature ranges 201–337, 337–638 and 638–982 °C. The CRTA technology enables the separation of the thermal decomposition steps.





Similar content being viewed by others
References
Caillere S, Henin S. The application of differential thermal analysis to the study of the clay minerals found in soils. Ann Agron. 1947;17:23–72.
Sudo T, Hayashi H. New types of clay minerals with long spacings at about 30 A. found from the altered area developed around certain ore bodies of the Hanaoka Mine, Akita Prefecture. Sci Rep Tokyo Kyoiku Daigaku C Geol Miner Geogr. 1955;3:281–94.
Koltermann M, Rauschenfels E, Pfefferkorn H. The thermal behavior of sepiolite. Tonind Ztg Keram Rundschau. 1964;88:132–4.
Murat M, Gielly J. Reactions of dehydration and dehydroxylation of clay minerals studies by electric conductivity measurements and differential thermal analysis. Bull Groupe Fr Argiles. 1969;21:151–76.
Hayashi H, Otsuka R, Imai N. Infrared study of sepiolite and palygorskite on heating. Am Mineral. 1969;54:1613–24.
Vicente MA, Suarez M, Lopez-Gonzalez JDD, Banares-Munoz MA. Characterization, surface area, and porosity analysis of the solids obtained by acid leaching of a saponite. Langmuir. 1996;12:566–72.
Kinoshita R, Ichimura Y, Onodera J, Niimura N, Nakayama Y, Higuchi T. The optimization of the TG/DTA-MS measurements and the application for the material analysis. J Mass Spectrom Soc Jpn. 1998;46:365–73.
Brigatti MF, Franchini GC, Medici L, Poppi L, Stewart A. Behavior of sepiolite in Co2+ Cu2+ and Cd2+ removal from a simulated pollutant solution. Ann Chim (Rome). 1998;88:461–70.
Yariv S, Lapides I. The effect of mechanochemical treatments on clay minerals and the mechanochemical adsorption of organic materials onto clay minerals. J Mater Synth Process. 2000;8:223–33.
Cebulak S, Langier-Kuzniarowa A. Some remarks on the methodology of thermal analysis of clay minerals. J Therm Anal Calorim. 1998;53:375–81.
Perez-Rodriguez JL, Galan E. Determination of impurity in sepiolite by thermal analysis. J Therm Anal. 1994;42:131–41.
Aramendia MA, Borau V, Corredor JI, Jimenez C, Marinas JM, Ruiz JR, et al. Characterization of the structure and catalytic activity of Pt/sepiolite catalysts. J Colloid Interface Sci. 2000;227:469–75.
Fernandez Alvarez T. Studies of pore structure of various solids by nitrogen adsorption. I. Natural sepiolites activated by hydrochloric acid. In: Proceedings of the international clay conference; 1973. p. 571–82.
Serna C, Ahlrichs JL, Serratosa JM. Folding in sepiolite crystals. Clays Clay Miner. 1975;23:452–7.
Serna C, Rautureau M, Prost R, Tchoubar C, Serrarosa JM. Study of sepiolite with the aid of data from electronic microscopy, thermogravimetric analysis, and infrared spectroscopy. Bulletin du Groupe Francais des Argiles. 1974;26:153–63.
Nagata H, Sudo T. Dehydration and dehydroxylation of sepiolite. In: Proceedings of the 5th international conference on thermal analysis; 1977. p. 534–7.
Yariv S. Combined DTA-mass spectrometry of organo-clay complexes. J Therm Anal. 1990;36:1953–61.
Serna C, Ahlrichs JL, Serratosa JM. Sepiolite anhydride and crystal folding. Clays Clay Miner. 1975;23:411–2.
Serna C, VanScoyoc GE, Ahlrichs JL. Hydroxyl groups and water in palygorskite. Am Mineral. 1977;62:784–92.
Serna C, VanScoyoc GE, Ahlrichs JL. Uncoupled water found in palygorskite. J Chem Phys. 1976;65:3389–90.
Frost RL, Erickson KL. Thermal decomposition of synthetic hydrotalcites reevesite and pyroaurite. J Therm Anal Calorim. 2004;76:217–25.
Horvath E, Kristof J, Frost RL, Heider N, Vagvoelgyi V. Investigation of IrO2/SnO2 thin film evolution by thermoanalytical and spectroscopic methods. J Therm Anal Calorim. 2004;78:687–95.
Frost RL, Weier ML, Erickson KL. Thermal decomposition of struvite. J Therm Anal Calorim. 2004;76:1025–33.
Frost RL, Erickson KL. Thermal decomposition of natural iowaite. J Therm Anal Calorim. 2004;78:367–73.
Horvath E, Kristof J, Frost RL, Redey A, Vagvolgyi V, Cseh T. Hydrazine-hydrate intercalated halloysite under controlled-rate thermal analysis conditions. J Therm Anal Calorim. 2003;71:707–14.
Kristof J, Frost RL, Kloprogge JT, Horvath E, Mako E. Detection of four different OH groups in ground kaolinite with controlled-rate thermal analysis. J Therm Anal Calorim. 2002;69:77–83.
Frost RL, Martens W, Ding Z, Kloprogge JT. DSC and high-resolution TG of synthesized hydrotalcites of Mg and Zn. J Therm Anal Calorim. 2003;71:429–38.
Bouzaid J, Frost RL. Thermal decomposition of stichtite. J Therm Anal Calorim. 2007;89:133–5.
Bouzaid JM, Frost RL, Martens WN. Thermal decomposition of the composite hydrotalcites of iowaite and woodallite. J Therm Anal Calorim. 2007;89:511–9.
Bouzaid JM, Frost RL, Musumeci AW, Martens WN. Thermal decomposition of the synthetic hydrotalcite woodallite. J Therm Anal Calorim. 2006;86:745–9.
Frost RL, Bouzaid JM, Musumeci AW, Kloprogge JT, Martens WN. Thermal decomposition of the synthetic hydrotalcite iowaite. J Therm Anal Calorim. 2006;86:437–41.
Frost RL, Ding Z, Ruan HD. Thermal analysis of goethite. Relevance to Australian indigenous art. J Therm Anal Calorim. 2003;71:783–97.
Frost RL, Erickson K, Weier M. Thermal treatment of moolooite. J Therm Anal Calorim. 2004;77:851–61.
Frost RL, Kristof J, Martens WN, Weier ML, Horvath E. Thermal decomposition of sabugalite. J Therm Anal Calorim. 2006;83:675–9.
Frost RL, Kristof J, Weier ML, Martens WN, Horvath E. Thermal decomposition of metatorbernite – a controlled rate thermal analysis study. J Therm Anal Calorim. 2005;79:721–5.
Frost RL, Martens W, Adebajo MO. Synthesis of the mixed oxide catalysts based upon the nickel-copper hydrotalcites of the type NixCu6-xCr2(OH)16(CO3)*4H2O. J Therm Anal Calorim. 2005;81:351–5.
Frost RL, Musumeci AW, Adebajo MO, Martens W. Using thermally activated hydrotalcite for the uptake of phosphate from aqueous media. J Therm Anal Calorim. 2007;89:95–9.
Frost RL, Musumeci AW, Kloprogge JT, Weier ML, Adebajo MO, Martens W. Thermal decomposition of hydrotalcite with hexacyanoferrate(II) and hexacyanoferrate(III) anions in the interlayer. J Therm Anal Calorim. 2006;86:205–9.
Frost RL, Weier ML. Thermal decomposition of humboldtine – a high resolution thermogravimetric and hot stage Raman spectroscopic study. J Therm Anal Calorim. 2004;75:277–91.
Frost RL, Weier ML, Martens W. Thermal decomposition of jarosites of potassium, sodium and lead. J Therm Anal Calorim. 2005;82:115–8.
Frost RL, Weier ML, Martens W. Thermal decomposition of liebigite: a high resolution thermogravimetric and hot-stage Raman spectroscopic study. J Therm Anal Calorim. 2005;82:373–81.
Frost RL, Wills R-A, Kloprogge JT, Martens W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J Therm Anal Calorim. 2006;84:489–96.
Frost RL, Wills R-A, Kloprogge JT, Martens WN. Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J Therm Anal Calorim. 2006;83:213–8.
Lin Y-H, Adebajo MO, Frost RL, Kloprogge JT. Thermogravimetric analysis of hydrotalcites based on the takovite formula NixZn6-xAl2(OH)16(CO3)·4H2O. J Therm Anal Calorim. 2005;81:83–9.
Musumeci AW, Silva GG, Martens WN, Waclawik ER, Frost RL. Thermal decomposition and electron microscopy studies of single-walled carbon nanotubes. J Therm Anal Calorim. 2007;88:885–91.
Xi Y, Martens W, He H, Frost RL. Thermogravimetric analysis of organoclays intercalated with the surfactant octadecyltrimethylammonium bromide. J Therm Anal Calorim. 2005;81:91–7.
Frost RL, Kristof J, Horvath E, Kloprogge JT. The modification of hydroxyl surfaces of formamide-intercalated kaolinites synthesized by controlled rate thermal analysis. J Colloid Interface Sci. 2001;239:126–33.
Frost RL, Kristof J, Horvath E, Kloprogge JT. Separation of adsorbed formamide and intercalated formamide using controlled rate thermal analysis methodology. Langmuir. 2001;17:3216–22.
Frost RL, Kristof J, Horvath E, Martens WN, Kloprogge JT. Complexity of intercalation of hydrazine into kaolinite – a controlled rate thermal analysis and drift spectroscopic study. J Colloid Interface Sci. 2002;251:350–9.
Ding Z, Frost RL. Controlled rate thermal analysis of nontronite. Thermochim Acta. 2002;389:185–93.
Frost RL, Ding Z. Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites. Thermochim Acta. 2003;397:119–28.
Frost RL, Kristof J, Ding Z, Horvath E. Controlled rate thermal analysis of formamide intercalated kaolinites. 2001 A Clay Odyssey, proceedings of the 12th international clay conference, Bahia Blanca, Argentina, 22–28 July 2001; 2003. p. 523–30.
Nagata H. Thermal analysis of sepiolite by means of DSC. Nendo Kagaku. 1977;17:1–11.
Acknowledgements
This research was supported by the Hungarian Scientific Research Fund (OTKA) under Grant No. K62175. The financial and infra-structure support of the Queensland University of Technology Inorganic Materials Research Program is gratefully acknowledged. One of the authors (LMD) is grateful to the CRC for polymers for a Masters scholarship.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Calculation of water content for Sepiolite, Nevada
-
Composition: Mg4Si6O15(OH)2 · xH2O
-
Removing water up to 347 °C: 20.30 mg that is 1.127 mmol
-
Remaining dehydrated mineral up to 347 °C: 132.01 mg that is 0.245 mmol
-
Molar mass of dehydrated mineral: 539.80 g mol−1
-
Calculation of x:
-
1 mol dehydrated mineral—x mol H2O
-
0.245 mol dehydrated mineral—1.127 mol H2O
-
x = 4.6–5 mol
-
-
Formula: Mg4Si6O15(OH)2 · 5H2O
-
Steps of water liberation according to the decomposition steps up to 347 °C:
-
1.
step: 3.45 mol
-
2.
step: 1.15 mol
-
1.
Calculation of water content for Sepiolite, Nairobi
-
Composition: Mg4Si6O15(OH)2 · xH2O
-
Removing water up to 352 °C: 26.40 mg that is 1.465 mmol
-
Remaining dehydrated mineral up to 352 °C: 170.49 mg that is 0.316 mmol
-
Molar mass of dehydrated mineral: 539.80 g mol−1
-
Calculation of x:
-
1 mol dehydrated mineral—x mol H2O
-
0.316 mol dehydrated mineral—1.465 mol H2O
-
x = 4.63–5 mol
-
-
Formula: Mg4Si6O15(OH)2 · 5H2O
-
Steps of water liberation according to the decomposition steps up to 352 °C:
-
1.
step: 3.53 mol
-
2.
step: 1.10 mol
-
1.
Rights and permissions
About this article
Cite this article
Frost, R.L., Kristóf, J. & Horváth, E. Controlled rate thermal analysis of sepiolite. J Therm Anal Calorim 98, 749–755 (2009). https://doi.org/10.1007/s10973-009-0201-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0201-6