Abstract
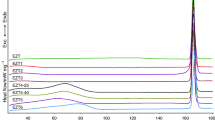
The physicochemical properties of theophylline hydrate and anhydrous polymorphic forms I and II were evaluated using crystallographic and calorimetric method. This study has been carried out with the following techniques: differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and X-ray diffractometry. The X-ray patterns on powder for investigated compounds are presented.




Similar content being viewed by others
References
Bettini R, Bertolini G, Frigo E, Rossi A, Casini I, Pasquali I, et al. Interaction of pharmaceutical hydrates with supercritical CO2. Solubility, solid-state and chemical modifications. J Therm Anal Calorim. 2004;77:625–38.
Barbas R, Prohens R, Puigjaner C. A new polymorph of norfloxacin. Complete characterization and relative stability of its trimorphic system. J Therm Anal Calorim. 2007;89:687–92.
Hilfiker R, Berghausen J, Blatter F, Burkhard A, De Paul SM, Freiermuth B, et al. Polymorphism integrated approach from high-throughput screening to crystallization optimization. J Therm Anal Calorim. 2003;73:429–40.
Legendre B, Feutelais Y. Polymorphic and thermodynamic study of indomethacin. J Therm Anal Calorim. 2004;76:255–64.
Griesser UJ, Szelagiewicz M, Hofmeier C, Pitt C, Cianferani S. Vapor pressure and heat of sublimation of crystal polymorphs. J Therm Anal Calorim. 1999;57:45–60.
Grčman M, Vrečer F, Meden A. Some physico-chemical properties of doxazosin mesylate polymorphic forms and its amorphous state. J Therm Anal Calorim. 2002;68:373–87.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68:335–57.
Giron D, Monnier S, Mutz M, Piechon P, Buser T, Stowasser F, et al. Comparison of quantitative methods for analysis of polyphasic pharmaceuticals. J Therm Anal Calorim. 2007;89:729–43.
Drebushchak VA, Drebushchak TN, Chukanov NV, Boldyreva EV. Transitions among five polymorphs of chlorpropamide near the melting point. J Therm Anal Calorim. 2008;93:343–51.
Perlovich GL, Volkova TV, Bauer-Brandl A. Polymorphism of paracetamol. Relative stability of the monoclinic and orthorhombic phase revisited by sublimation and solution calorimetry. J Therm Anal Calorim. 2007;89:767–74.
Schmidt AC, Niederwanger V, Griesser UJ. Solid-state forms of prilocaine hydrochloride crystal polymorphism of local anaesthetic drugs, Part II. J Therm Anal Calorim. 2004;77:639–52.
Caira MR, Bourne SA, Oliver CL. Thermal and structural characterization of two polymorphs of the bronchodilator tulobuterol. J Therm Anal Calorim. 2004;77:597–605.
Caira MR, Foppoli A, Sangalli ME, Zem L, Giordano F. Thermal and structural properties of ambroxol polymorphs. J Therm Anal Calorim. 2004;77:653–62.
Sun Ch, Zhou D, Grant D, Young VG. Theophylline monohydrate. Acta Cryst. 2002;58:368–70.
Bán M, Bombicz P, Madarász J. Thermal stability and structure of a new co-crystal of theophylline formed with phthalic acid TG/DTA-EGA-MS and TG-EGA-FTIR study. J Therm Anal Calorim. 2009;95:895–901.
Wesolowski M, Szynkaruk P. Thermal decomposition of methylxanthines interpretation of the results by PCA. J Therm Anal Calorim. 2008;93:739–46.
Suzuki E, Shimomura K, Sekiguchi K. Thermochemical study of theophylline and its hydrate. Chem Pharm Bull. 1989;37:493–97.
Legendre B, Randzio SL. Transitiometric analysis of solid II/solid I transition in anhydrous theophylline. Int J Pharm. 2007;343:41–7.
Legendre B, Baziard-Mouysset G, Anastassiadou M, Leger JM, Payard M. Polymorphic study of 2-(2-benzofuryl) Δ-2 imidazoline. J Therm Anal Calorim. 2001;66:659–73.
Note of Editor: Melting points of the elements. Bull Alloy Phase Diagr. 1986;7:601–2.
Wang P. ICDD Grant-in-Aid. Brooklyn: Polytechnic Institute of New York; 1975.
Li Y. ICDD Grant-in-Aid. New York: Polytechnic Institute of Brooklyn; 1972.
Ebisuzaki Y, Boyle PD, Smith AS. Methylxanthines. I. Anhydrous theophylline. Acta Cryst. 1997;53:777–9.
Sutor DJ. The structures of the pyrimidines and purines. VI. The crystal structure of theophylline. Acta Cryst. 1958;11:83–7.
Otsuka M, Kaneniwa N, Kawakami K, Umezawa O. Effect of surface characteristics of theophylline anhydrate powder on hygroscopic stability. J Pharm Pharmacol. 1990;42:606–10.
Doser H. Die Schmelzpunkte des Pantokains, Bromurals und Theophyllins. Arch Pharm. 1943;53:251–6. (in German).
Phadnis NV, Suryanarayanan R. Polymorphism in anhydrous theophylline-implications on the dissolution rate of theophylline tablets. J Pharm Sci. 1997;86:1256–63.
Burger A, Ramberger RA. On the polymorphism of pharmaceuticals and other molecular crystals. I, Theory of thermodynamic rules. Mikrochim Acta. 1979;2:259–71.
Yu L. Inferring thermodynamic stability relationship of polymorphs from melting data. J Pharm Sci. 1995;84:966–74.
Burger A, Ramberger R. Ramberger R. On the polymorphism of pharmaceuticals and other molecular crystals. II. Mikrochim Acta. 1979;256:273–316.
Acknowledgements
The authors are grateful to Prof. S. L. Randzio for the preparation of investigated compounds. The author Piotr Szterner would like to thank the Centre of Excellence TALES (Thermodynamic Laboratory for Environmental Purposes) for the support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szterner, P., Legendre, B. & Sghaier, M. Thermodynamic properties of polymorphic forms of theophylline. Part I: DSC, TG, X-ray study. J Therm Anal Calorim 99, 325–335 (2010). https://doi.org/10.1007/s10973-009-0186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0186-1