Abstract
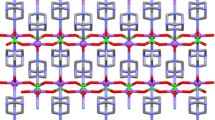
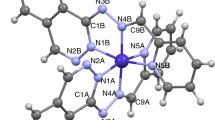
The new hydrazinium lanthanide metal complexes of 2-pyrazinecarboxylic acid (HpyzCOO) of the formulae (N2H5)2[Ln(pyzCOO)5] · 2H2O (1), where Ln = La or Ce and (N2H5)3[Ln(pyzCOO)4(H2O)] · 2NO3 (2), where Ln = Pr, Nd, Sm or Dy have been synthesized and characterized by physico-chemical methods. The IR absorption bands of N–N stretching at 960 cm−1 unambiguously prove the existence of N2H5 + ions. The bonding parameters β, b1/2, % δ and η, have been calculated from the electronic spectroscopic (hypersensitive) bands of Pr(III) and Nd(III) complexes. All the complexes undergo endothermic followed by exothermic decomposition to leave the respective metal oxides as the end products. However, the DTA of the complexes 2 demonstrate rather sharp peak than the complexes 1, owing to overwhelming exothermicity, which may be due to the loss of both hydrazine and nitrate moieties in the same step. The X-ray powder diffraction studies reveal the existence of isomorphism among the member complexes.




Similar content being viewed by others
References
O’Connor CJ, Klein CL, Majeste RJ, Trefonas LM. Magnetic properties and crystal structure of (2,3-pyrazinedicarboxylato)copper(II) hydrochloride: a pyrazine bridged ferromagnetic linear chain. Inorg Chem. 1982;21:64–7.
Drew MGB, Matthews RW, Walton RA. Studies on co-ordination complexes of silver(II). Part VI. Crystal and molecular structure of bis(pyridine-2,3-dicarboxylato)silver(II) dihydrate. J Chem Soc (A). 1971;2959–62.
Ptasiewicz-Bak H, Leciejewicz J. Crystal and molecular structures of nickel(II) complexes with pyrazine-2,3-dicarboxylic and 3-aminopyrazine-2-carboxylic acids. Polish J Chem. 1999;73:717–26.
Bayon JC, Eseban P, Net G, Rasmussen PG, Baker KN, Hahn CW, et al. Dinuclear platinum(II), palladium(II), nickel(II), and copper(II) complexes of 3,5-pyrazoledicarboxylic acid. Inorg Chem. 1991;30:2572–4.
Klein CL, Majeste RJ, Trefonas LM, O’conner CJ. Magnetic properties and molecular structure of copper(II) complexes of pyrazinecarboxylic acid. Inorg Chem. 1982;21:1891–7.
O’Connor CJ, Sinn E. Crystal structures and magnetic properties of cobalt(II) pyrazinecarboxylate and pyrazinedicarboxylate complexes. Inorg Chem. 1981;20:545–51.
Ptasiewicz-Bak H, Leciejewicz J, Zachora J. X-ray structure analysis of diaquobis(2-pyrazinecarboxylato) manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II) complexes. J Coord Chem. 1995;36:317–26.
Alcock NW, Kemp TJ, Roe SM, Leciejewicz J. The roles of N- and O-coordination in the crystal and molecular structures of uranyl complexes with anthranilic and pyrazinic acids. Inorg Chim Acta. 1996;248:241–6.
Ptasiewicz-Bak H, Ostrowsk A, Leciejewicz J. Monomeric molecules in the isostructural calcium(II) and strontium(II) complexes with pyrazine-2-carboxylic acid. Polish J Chem. 1998;72:2014–23.
Premkumar T, Govindarajan S. Thermoanalytical and spectral properties of new rare-earth metal 2-pyrazinecarboxylate hydrates. J Therm Anal Cal. 2005;79:685–9.
Burgess BK, Wherland S, Stiefel EI, Newton WE. Nitrogenase reactivity: insight into the nitrogen-fixing process through hydrogen-inhibition and HD-forming reactions. Biochemistry. 1981;20:5140–6.
Davis LC. Hydrazine as a substrate and inhibitor of Azotobacter vinelandii nitrogenase. Arch Biochem Biophys. 1980;204:270–6.
Dilworh MJ, Eady RR. Hydrazine is a product of dinitrogen reduction by the vanadium-nitrogenase from Azotobacter chroococcum. Biochem J. 1991;277:465–8.
Evans DJ, Henderson RA, Smith BE. In: Reedijk J, editor. Bioinorganic catalysis. New York: Marcel Dekker; 1993.
Moeller T, Brinhaum ER, Frosberg JH, Gayhart RB, Eyring L, editors. Progress in science and technology of the rare earths, vol. 3. Oxford, London: Pergamon Press; 1968.
Schmidt EW. Hydrazine and its derivatives. New York: Wiley/Interscience; 1984.
Alive RY, Musaev DB. Zh Obshch Khim. 1976;46:9.
Bezdenezhnykh GV, Sharov VA, Krylov EI. Russ J Inorg Chem. 1974;19:1122.
Kuppusamy K, Govindarajan S. New trivalent lanthanide complexes of phthalate-containing hydrazinium cation – preparation, and spectral and thermal studies. Thermochim Acta. 1996;279:143–55.
Govindarajan S, Patil KC, Manohar H, Werner PE. Hydrazinium as a ligand: structural, thermal, spectroscopic, and magnetic studies of hydrazinium lanthanide di-sulphate monohydrates; crystal structure of the neodymium compound. J Chem Soc Dalton Trans. 1986;1:119–23.
Bokovec N, Milicev S. Synthesis, powder data, infrared and Raman spectra of N2H5Ln(SO4)2·H2O (Ln = La—Tb, except Pm). Inorg Chim Acta. 1987;128:L25–L28.
Govindarajan S, Patil KC, Poojary MD, Manohar H. Synthesis, characterization and X-ray structure of hexahydrazinium diuranyl pentaoxalate dihydrate, (N2H5)6(UO2)2(C2O4)5·2H2O. Inorg Chim Acta. 1986;120:103–7.
Premkumar T, Govindarajan S. The chemistry of hydrazine derivatives—thermal behavior and characterisation of hydrazinium salts and metal hydrazine complexes of 4,5-imidazoledicarboxylic acid. Thermochim Acta. 2002;386:35–42.
Premkumar T, Govindarajan S, Pan W-P. Preparation, spectral and thermal studies of pyrazinecarboxylic acids and their hydrazinium salts. Proc Indian Acad Sci (Chem Sci). 2003;115:103–11.
Premkumar T, Govindarajan S. Synthesis and spectroscopic, thermal, and XRD studies on trivalent lighter rare-earth complexes of 2,3-pyrazinedicorboxylate with hydrazinium cation. Inorg Chem Commun. 2003;6:1385–9.
Premkumar T, Govindarajan S, Coles AE, Wight CA. Thermal decomposition kinetics of hydrazinium cerium 2,3-pyrazinedicarboxylate hydrate: a new precursor for CeO2. J Phys Chem B. 2005;109:6126–9.
Premkumar T, Govindarajan S, Xie R, Pan W-P. Preparation and thermal behaviour of transition metal complexes of 4,5-imidazoledicarboxylic acid. J Therm Anal Cal. 2003;74:325–33.
Premkumar T, Govindarajan S. Transition metal complexes of pyrazinecarboxylic acids with neutral hydrazine as a ligand. J Therm Anal Cal. 2005;79:115–21.
Premkumar T, Govindarajan S. Divalent transition metal complexes of 3,5-pyrazoledicarboxylate. J Therm Anal Cal. 2006;84:395–9.
Leciejewicz J, Ptasiewicz-Bak H, Premkumar T, Govindarajan S. Crystal structure of a lanthanum(III) complex with pyrazine-2-carboxylate and water ligands. J Coord Chem. 2004;57:97–103.
Vogel AI. A text book of quantitative inorganic analysis. 4th ed. London: Longman; 1986.
Cariati F, Naldini I, Panzanelli A, Demartin F, Manasseso M. Bis(triphenylphosphine)pyridine- and pyrazine-carboxylatecopper(I) complexes. Inorg Chim Acta. 1983;69:117–22.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 3rd ed. New York: Wiley Interscience; 1978.
Lord RC, Martson AL, Miller FA. Infra-red and Raman spectra of the diazines. Spectrochim Acta. 1957;9:113–25.
Allan JR, Baird ND, Kassyk AL. Some first row transition metal complexes of nicotinamide and nicotinic acid. J Thermal Anal. 1979;16:79–90.
Lever ABP. Inorganic electronic spectroscopy. 2nd ed. Amsterdam: Elsevier; 1984.
Carnall WT, Fields PR, Wybourne BG. Spectral intensities of the trivalent lanthanides and actinides in solution. I. Pr3+, Nd3+, Er3+, Tm3+, and Yb3+. J Chem Phys. 1965;42:3797–806.
Choppin GR, Henrie DE, Buijs K. Environmental effects on f–f transitions. I. Neodymium(III). Inorg Chem. 1966;5:1743–8.
Karraker DG. Hypersensitive transitions of six-, seven-, and eight-coordinate neodymium, holmium, and erbium chelates. Inorg Chem. 1967;6:1863–8.
Sinha SP. Spectroscopic investigations of some neodymium complexes. Spectrochim Acta. 1966;22:57–62.
Jorgensen CK. Modern aspects of the ligand field theory. North Holland; 1971.
Sinha SP. 2,2′-Dipyridyl complexes of rare earths—II: reflection spectra of Nd(III)-bis-(2,2′-dipyridyl) and Nd(III)-bis-(4,4′-dimethyl-2,2′-dipyridyl) chlorides. J Inorg Nucl Chem. 1965;27:115–8.
Premkumar T, Govindarajan S, Rath NP, Manivannan V. New nine and ten coordinated complexes of lanthanides with bidentate 2-pyrazinecarboxylate containing hydrazinium cation. Inorg Chim Acta. 2009;362:2941–6.
Acknowledgements
T. Premkumar thanks the Council of Scientific and Industrial Research, New Delhi, for the award of a Senior Research Fellowship. The authors gratefully acknowledge Dr. Nigam P. Rath, Department of Chemistry and Biochemistry, University of Missouri-St. Louis, USA and Dr. J. W. Steed, Kings College, London for helping with single crystal X-ray data collection for the compounds.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Premkumar, T., Govindarajan, S. Thermoanalytical and spectroscopic studies on hydrazinium lighter lanthanide complexes of 2-pyrazinecarboxylic acid. J Therm Anal Calorim 100, 725–732 (2010). https://doi.org/10.1007/s10973-009-0117-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0117-1