Abstract
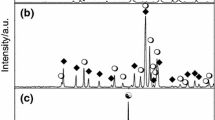
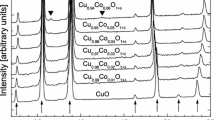
In this work it has been established which compounds finally are formed in air in the two-component CuO-V2O5 and CuO-α-Sb2O4 systems. Unknown thermal properties of CuV2O6, Cu2V2O7 and Cu11V6O26 have been established. Reactivity of the oxides and phase relations in the ternary V2O5-CuO-α-Sb2O4 system in air have been studied by using XRD and DTA methods. The results have showed the reaction of V2O5, CuO with α-Sb2O4 does not produce any compound where all the three oxides would be involved. It has been established that the α-Sb2O4 reacts and forms binary phases independently with CuO or V2O5. On the base of these results the investigated system was divided into subsidiary subsystem in which CuSb2O6 remains at equilibrium in the solid state with other phases formed in corresponding binary systems.
Similar content being viewed by others
References
J. Sambeth, A. Juan, L. Gambaro and H. Thomas, J. Mol. Catal., 118 (1997) 283.
S. Pak, C. E. Smith and M. Rosynek, J. Catal., 165 (1997) 73.
J. P. Olenkowa and L. M. Plasowa, Z. Struct. Khim., 19 (1978) 1040.
R. B. Zohn, M. Reny, T. Machej and B. Delmon, Catal. Today, 1 (1987) 181.
T. Brichall and A. Sleight, Inorg. Chem., 15 (1976) 868.
F. J. Berry and M. E. Brett, Inorg. Chim. Acta, 87 (1984) 133.
S. Hansen, K. Stahl, R. Wilson and A. Andersons, J. Solid State Chem., 102 (1993) 340.
A. Canavals, J. Nilsson, S. Hansen, K. Stahl and A. Andersons, J. Solid State Chem., 116 (1995) 369.
F. J. Berry, M. E. Brett and R. A. Marbrov, J. Chem. Soc., Dalton Trans., (1984) 985.
E. Filipek, Solid State Sci., 8 (2006) 577.
M. Stan, S. Shimadu, D. Crisan and M. Zacharescu, Eur. J. Solid State Inorg. Chem., 35 (1998) 243.
S. Shimada, K. Kodaira and T. Matsushita, J. Solid State Chem., 59 (1985) 237.
S. Shimada and K. J. D. Mackenzie, Thermochim. Acta, 56 (1982) 73.
T. N. Kol’tsova and A. E. Chastukhin, Inorg. Mater., 38 (2002) 1228.
O. Scarlat, M. Susana-Mihaiu and M. Zaharescu, J. Eur. Ceram. Soc., 22 (2002) 1839.
A. V. Prokofiev, F. Ritter, W. Assmus, B. J. Gibson and R. K. Kremer, J. Cryst. Growth, 247 (2003) 457.
P. Fleury, C. R. Acad. Sci., 263 (1966) 1375.
A. Brisi and A. Molinari, Ann. Chim. (Rome), 48 (1958) 263.
M. Hughes and C. G. Hadidiacos, Am. Mineral., 70 (1985) 193.
J. Coing Boyat, Acta. Cryst., B38 (1982) 1546.
D. Mercurio-Lavaud and B. Frit, Acta Cryst., B29 (1973) 2737.
D. Mercurio-Lavaud and B. Frit, C. R. Acad. Sci. Paris, 277 (1973) 1101.
D. Mercurio-Lavaud, J. Gally and B. Frit, C. R. Acad. Sci. Paris, 282 (1976) 27.
G. M. Clark and R. Garlick, J. Inorg. Nucl. Chem., 44 (1978) 1347.
D. Lavaud and J. Galy, Bull. Soc. Fr. Mineral. Cristallogr., 95 (1972) 134.
A. V. Prokofiev, R. K. Kremer and W. Assmus, J. Cryst. Crowth, 231 (2001) 498.
C. Calvo and D. Manolescu, Acta Cryst., B29 (1973) 1743.
P. Fleury, Rev. Chim. Min., 6 (1969) 819.
V. Cirilli, A. Burdese and C. Brisi, Atti. Acad. Sci. Torino, 95 (1961) 15
H. P. Christian and H. Mueller-Buschbaum, Z. Naturforsch. B, 30 (1975) 175.
J. R. Rea and E. Kostiner, J. Solid State Chem., 7 (1973) 17.
E. Filipek, The Synthesis and Physicochemical Properties of New Phases in the Systems Oxides V2O5, MoO3, α-Sb2O4 (Polish), Editorial office-Szczecin University of Technology, 2007, ISBN 978-83-7457-025-1.
J. Walczak, J. Ziółkowski, M. Kurzawa and J. Osten-Sacken, Pol. J. Chem., 59 (1985) 255.
F. A. Cotton, G. Wilkinson and P. L. Gaus, Basic Inorganic Chemistry, PWN, 1998.
P. Belina and P. Sulcova, J. Therm. Anal. Cal., 88 (2007) 107.
K. Wieczorek-Ciurowa and K. Gamrat, J. Therm. Anal. Cal., 88 (2007) 213.
Diffraction File, International Center for Diffraction data, Swarthmore (USA)1989, File Nos. 80-0076; 80-0231; 9-387; 81-0422; 45-1054; 73-0189; 70-0831; 26-0569; 74-1503; 74-1401; 36-0431; 26-0570; 70-1696.
E. Filipek, Solid State Sci., 8 (2006) 577.
A. Błońska-Tabero, J. Therm. Anal. Cal., 88 (2007) 562.
P. Tabero, J. Therm. Anal. Cal., 88 (2007) 562.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dąbrowska, G., Filipek, E. Reactivity of the oxides in the ternary V2O5-CuO-α-Sb2O4 system in air. J Therm Anal Calorim 93, 839–845 (2008). https://doi.org/10.1007/s10973-008-9301-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9301-y