Abstract
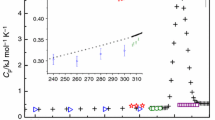
Heat capacity and enthalpy increments of calcium niobates CaNb2O6 and Ca2Nb2O7 were measured by the relaxation time method (2–300 K), DSC (260–360 K) and drop calorimetry (669–1421 K). Temperature dependencies of the molar heat capacity in the form C pm=200.4+0.03432T−3.450·106/T 2 J K−1 mol−1 for CaNb2O6 and C pm=257.2+0.03621T−4.435·106/T 2 J K−1 mol−1 for Ca2Nb2O7 were derived by the least-squares method from the experimental data. The molar entropies at 298.15 K, S 0m (CaNb2O6, 298.15 K)=167.3±0.9 J K−1 mol−1 and S 0m (Ca2Nb2O7, 298.15 K)=212.4±1.2 J K−1 mol−1, were evaluated from the low temperature heat capacity measurements. Standard enthalpies of formation at 298.15 K were derived using published values of Gibbs energy of formation and presented heat capacity and entropy data: Δf H 0(CaNb2O6, 298.15 K)= −2664.52 kJ molt-1 and Δf H 0(Ca2Nb2O7, 298.15 K)= −3346.91 kJ mol−1.
Similar content being viewed by others
References
M. Ibrahim, N. F. H. Bright and J. F. Rowland, J. Am. Ceram. Soc., 45 (1962) 329.
A. Jongejan, J. Less-Common Met., 19 (1969) 193.
T. A. Vanderah, W. Febo, J. Y. Chan, R. S. Roth, J. M. Loezos, L. D. Rotter, R. G. Geyer and D. B. Minor, J. Solid State Chem., 155 (2000) 78.
V. G. Dneprova, T. N. Rezukhina and Ya. I. Gerasimov, Doklady Akad. Nauk SSSR, 178 (1968) 135.
O. Kubaschewski, High Temp. — High Pressures, 4 (1972) 1.
O. Knacke, O. Kubaschewski and K. Hesselmann (Eds.), Thermochemical Properties of Inorganic Substances, 2nd Ed., Springer, Berlin 1991, p. 384.
S. Raghavan, Trans. Indian Inst. Met., 44 (1991) 285.
S. Raghavan, J. Alloys Compd., 179 (1992) L25.
P. Abrman, D. Sedmidubský, A. Strejc, P. Voňka and J. Leitner, Thermochim. Acta, 381 (2002) 1.
M. Hampl, A. Strejc, D. Sedmidubský, K. Růžička, J. Hejtmánek and J. Leitner, J. Solid State Chem., 179 (2006) 77.
M. Hampl, J. Leitner, K. K. Růžička, M. Straka and P. Svoboda, J. Therm. Anal. Cal., 87 (2007) 553.
J. Leitner, M. Hampl, K. K. Růžička, D. Sedmidubský, P. Svoboda and J. Vejpravová, Thermochim. Acta, 450 (2006) 105.
J. Leitner, M. Hampl, K. K. K. Růžička, M. Straka, D. Sedmidubský and P. Svoboda, J. Therm. Anal. Cal., 91 (2008) 985.
J. S. Hwang, K. J. Lin and C. Tien, Rev. Sci. Instrum., 68 (1997) 94.
W. Schnelle, J. Engelhardt and E. Gmelin, Cryogenics, 39 (1999) 271.
Quantum Design, Physical Property Measurement System — Application Note, http://www.qdusa.com/pdf/brochures/heat.pdf.
J. W. Arblaster, Platinum Metals Rev., 38 (1994) 119.
J. P. Cummings and S. H. Simonsen, Am. Miner., 55 (1970) 90.
R. M. Rakhmankulov and Yu. P. Udalov, Zh. Neorg. Khim., 21 (1976) 2842.
V. I. Spitsyn, E. A. Ippolitova, L. M. Kovba, L. N. Lykova and P. P. Leshchenko, Zh. Neorg. Khim., 27 (1982) 827.
N. Ishizawa, F. Marumo, S. Iwai, M. Kimura and T. Kawamura, Acta Cryst., B36 (1980) 763.
C. A. Martín, J. Phys. Condens. Matter, 3 (1991) 5967.
W. H. Press, S. A. Teukolsky, W. T. Vetterling and B. P. Flannery, Numerical Recipes in FORTRAN, 2nd Ed., Ch. 10.4, pp. 402–406, Cambridge University Press, 1992.
J. Leitner, P. Chuchvalec, D. Sedmidubský, A. Strejc and P. Abrman, Thermochim. Acta, 395 (2003) 27.
J. R. Taylor and A. T. Dinsdale, CALPHAD, 14 (1990) 71.
P. Richet and G. Fiquet, J. Geophys. Res., 96 (1991) 445.
L. Qiu and M. A. White, J. Chem. Educ., 78 (2001) 1076.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leitner, J., Růžička, K., Sedmidubský, D. et al. Heat capacity, enthalpy and entropy of calcium niobates. J Therm Anal Calorim 95, 397–402 (2009). https://doi.org/10.1007/s10973-008-9245-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9245-2