Abstract
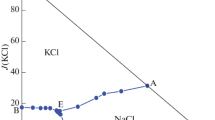
The solubility and the physicochemical properties (densities, viscosities, refractive indices, conductivities and pH) in the liquid-solid metastable system (NaCl-KCl-CaCl2-H2O) at 308.15 K have been investigated using the isothermal evaporation method, and the dry-salt phase diagram, water-phase diagram, and the diagram of physicochemical properties vs. composition in the system were plotted. One three-salt cosaturated point, three metastable solubility isotherm curves, and three crystallization regions corresponding to sodium chloride, potassium chloride and calcium chloride tetrahydrate were formed, and neither solid solution nor double salts were found. On the basis of the extended Harvie-Weare (HW) model and its temperature-dependent equation, the values of the Pitzer parameters β(0), β(1), C ϕ for NaCl, KCl and CaCl2, the mixing ion-interaction parameters θNa,K, θNa,Ca, θK,Ca, ΨNa,K,Cl, ΨNa,Ca,Cl, ΨK,Ca,Cl, and the Debye-Hückel parameter A ϕ and the chemical potentials of the minerals in the quaternary system at 308.15 K were fitted, and the predictive solubility based on the temperature-dependent equation and the chemical potentials of the minerals agrees well with the experimental data.
Similar content being viewed by others
References
J. L. Fu, S. S. Yu, S. J. Li and H. Y. Ren, J. Salt Lake Res., 13 (2005) 17.
X. Y. Zheng, Y. Tang and C. Xu, Tibet Saline Lake. Chin. Sci. Press, Beijing (1988).
T. L. Deng, D. C. Li and S. Q. Wang, J. Chem. Eng. Data, 53 (2008) 1007.
V. P. Il’inskii, N. A. Varypaev, K. E. Gitterman and N. E. Shmidt, Tr. Solyanoi Lab., Akad. Nauk SSSR, 7 (1936) 32.
E. I. Luk’yanova and D. N. Shoikhet, Tr. Gos. Inst. Prikl. Khim., 34 (1940) 16.
O. K. Yanat’eva, Zh. Obshch. Khim., 17 (1947) 1040.
G. O. Assarsson, J. Am. Chem. Soc., 72 (1950) 1437 and 1440.
T. Mayer, C. Prutton and C. W. Lightfoot, J. Am. Chem. Soc., 71 (1949) 1237.
Analytical laboratory of Qinghai Institute of Salt Lakes at CAS, The Analyses of Brines and Salts, 2nd Ed. Chin. Sci. Press, Beijing 1988.
K. S. Pitzer, J. Phys. Chem., 77 (1973) 268.
K. S. Pitzer, Semi-empirical equations for pure and mixed electrolytes. Thermodynamics, 3rd Ed. McGraw-Hill, New York 1995.
C. E. Harvie and J. H. Weare, Geochim. Cosmochim. Acta, 44 (1980) 981.
C. E. Harvie, N. Moller and J. H. Weare, Geochim. Cosmochim. Acta, 48 (1984) 723.
R. T. Pabalan and K. S. Pitzer, Geochim. Cosmochim. Acta, 51 (1987) 2429.
N. Moller, Geochim. Cosmochim. Acta, 52 (1988) 821.
J. P. Greenberg and N. Møller, Geochim. Cosmochim. Acta, 53 (1989) 2503.
R. J. Spencer, N. Moller and J. Weare, Geochim. Cosmochim. Acta, 54 (1990) 575.
A. R. Nazmi, T. Reinisch and H. J. Hinz, J. Therm. Anal. Cal., 91 (2008) 141.
C. Christov and N. Møller, Geochim. Cosmochim. Acta, 68 (2004) 3717.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D.C., Deng, T.L. Liquid-solid metastable phase equilibria for the quaternary system (NaCl-KCl-CaCl2-H2O) at 308.15 K. J Therm Anal Calorim 95, 361–367 (2009). https://doi.org/10.1007/s10973-008-9236-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9236-3