Abstract
Organic peroxides are commonly employed as an initiator for polymerization, a source of free radicals, a hardener, and a linking agent. Due to its relatively weak oxygen-oxygen bond, di-tert butyl peroxide (DTBP) has been categorized as flammable type or Class III by the National Fire Protection Association (NFPA). The transport of dangerous goods (TDG) has published a warning against DTBP that it could potentially induce violent heat, explosion, fire and self-ignition under certain circumstances.
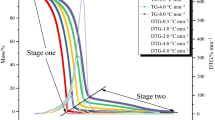
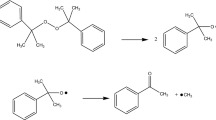
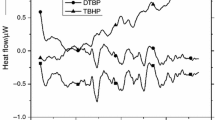
DTBP has been recommended as an international standard sample for estimating the performance of several calorimeters, such as glass tube tests, differential scanning calorimetry (DSC), and vent sizing package 2 (VSP2). In this study, we measured the precise temperature changes and heat flow with the above-mentioned testing instruments. However, some runaway incidents caused by DTBP have demonstrated the reaction temperature could be as low as ambient temperature. The reactivity and the hazardous incompatibility with sulfuric acid (H2SO4) and hydrochloric acid (HCl) of DTBP have not been evident, and the runaway hazards involved in different processing conditions were clarified in this study by implementing the two calorimeters. Acid-catalyzed characteristics and reaction hazards of DTBP could be acquired, such as heat of decomposition (ΔH d) and exothermic onset temperature (T 0).
Similar content being viewed by others
References
Y. Ilzuko and M. Surianarayana, Ind. Eng. Chem. Res., 42 (2003) 298.
T. K. Kang and C. S. Ha, Polym. Test., 19 (2000) 773.
X. R. Li and H. Koseki, J. Loss Prev. Process Ind., 18 (2005) 460.
Thermal Hazard Technology, Technical Information Sheet No. 100, England.
R. J. Kersten, M. N. Boers, M. M. Stork and C. Visser, J. Loss Prev. Process Ind., 18 (2005) 145.
R. F. Antrim, M. T. Bender, M. B. Clark, L. Evers, D. C. Hendershot, J. W. Magee, M. McGeorge, P. C. Morton, J. G. Nelson and C. Q. Zeszotarski, American Institute of Chemical Engineers, 32nd Annual Loss Prevention Symposium, 1998.
A. Miyake, N. Yanada and Y. Ogawa, J. Loss Prev. Process Ind., 18 (2005) 380.
J. M. Tseng, C. M. Shu, J. J. Horng, C. M. Kuan and H. I. Hsu, Process Saf. Environ Prot., 85 (2007) 125.
R. H. Chang, J. M. Tseng, J. M. Jehng, C. M. Shu and H. Y. Hou, J. Therm. Anal. Cal., 83 (2006) 57.
M. H. Yuan, C. M. Shu and A. A. Kossoy, Thermochim. Acta, 430 (2005) 67.
H. Y. Hou, T. S. Liao, Y. S. Duh and C. M. Shu, J. Therm. Anal. Cal., 83 (2006) 167.
D. D. MacNeil, S. Trussler, H. Fortier and J. R. Dahn, Thermochim. Acta, 386 (2002) 153.
Mettler Toledo, STARe Software with Solaris Operating System, Operating Instructions, Switzerland: Mettler Toledo, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, R.P., Hou, H.Y., Tseng, J.M. et al. Reactive incompatibility of DTBP mixed with two acid solutions. J Therm Anal Calorim 93, 269–274 (2008). https://doi.org/10.1007/s10973-007-8832-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-007-8832-y