Abstract
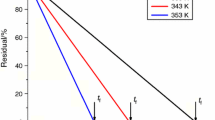
An isothermal dehydration of equilibrium swollen poly(acrylic acid) hydrogel in the temperature range from 306 to 361 K was investigated. The specific parameters connected with shape of the conversion curves were defined. The activation parameters (E, lnA) of the isothermal dehydration of equilibrium swollen poly(acrylic acid) hydrogel were calculated, using Johnson-Mehl-Avrami (JMA), ‘initial rate’ and ’stationary point’ methods. The reaction models for the investigated dehydration are determined using the ‘model-fitting’ method. It was established that both, the reaction model and activation parameters of the hydrogel dehydration were completely different for the isothermal process than for the non-isothermal one. It was found that the increase in dehydration temperature lead to the changes in isothermal kinetic model for the investigated hydrogel dehydration. It was established that the apparent activation energy (E) of hydrogel dehydration is similar to the value of the molar enthalpy of water evaporation.
Similar content being viewed by others
References
N. Peppas, Hydrogels in Medicine and Pharmacy, Vol. I: Fundamentals, CRC, Boca Raton, FL 1986.
E. Karadağ and D. Saraydin, Polym. Bull., 48 (2002) 299.
D. Saraydin and Y. Caldiran, Polym. Bull., 46 (2001) 91.
I. Katime, J.L. Velada, R. Novoa, E. Diaz de Apodaca, J. Puig and E. Mendizabal, Polym. Intern., 40 (1996) 281.
E. Karadağ and D. Saraydin, Turk. J. Chem., 26 (2002) 863.
P. M. de la Torre, S. Torrado and S. Torrado, Biomaterials, 24 (2003) 1459.
B. Janković, B. Adnağević and J. Jovanović, J. Therm. Anal. Cal., 82 (2005) 7.
B. Janković, B. Adnağević and J. Jovanović, Thermochim. Acta, 452 (2007) 106.
J. Jovanović and B. Adnağević, XLI Consultation of Serbian Chemical Society, SSHD, Belgrade, 23–24 January, Book of Abstracts, HM13, 2003, p. 119.
W. A. Johnson and R. F. Mehl, Trans. Am. Inst. Miner. (Metall.) Eng., 135 (1939) 416.
M. Avrami, J. Chem. Phys., 7 (1939) 1103.
I. Klarić, U. Roje and T. Kovačić, J. Therm. Anal. Cal., 45 (1995) 1373.
J. D. Hancock and J. H. Sharp, J. Amer. Ceram. Soc., 55 (1972) 74.
M. E. Brown, D. Dollimore and A. K. Galwey, Reactions in the Solid State in Comprehensive Chemical Kinetics, H. Bamford, C. F. H. Tipper, Eds, Elsevier, Amsterdam 1980, Vol. 22.
S. Vyazovkin and C. A. Wight, Thermochim. Acta, 340–341 (1999) 53.
S. Vyazovkin, J. S. Clawson and C. A. Wight, Chem. Mater., 13 (2001) 960.
S. Vyazovkin and C. A. Wight, Int. Rev. Phys. Chem., 17 (1998) 407.
F. Rodante, G. Catalani and S. Vecchio, J. Therm. Anal. Cal., 68 (2002) 689.
S. Vyazovkin and C. A. Wight, J. Phys. Chem. A, 101 (1997) 8279.
M. L. Franklin and T. B. Flanagan, J. Phys. Chem., 75 (1971) 1272.
S. F. Hilbert, J. Br. Ceram. Soc., 6 (1969) 11.
R. F. Walker, Trans. Faraday Soc., 65 (1969) 3324.
R. C. Eckhardt and T. B. Flanagan, Trans. Faraday Soc., 60 (1964) 1289.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janković, B., Adnađević, B. & Jovanović, J. Isothermal kinetics of dehydration of equilibrium swollen poly(acrylic acid) hydrogel. J Therm Anal Calorim 92, 821–827 (2008). https://doi.org/10.1007/s10973-007-7558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-007-7558-1