Abstract
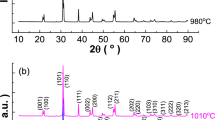
In order to obtain pure and fine BaTiO3 powders with controlled morphology, sol-precipitation methods involving the use of titanium iso-propoxide and of two different barium sources, i.e. barium nitrate and barium acetate, were proposed in this work. The thermal behaviour of the synthesized gels and the X-ray diffraction data obtained for the oxide powders pointed out that, by using Ba(NO3)2 as barium source, the decomposition process was completed at lower temperature (750°C) and was accompanied by a more pronounced tendency to obtain a single phase BaTiO3 composition, by comparison with the synthesis where barium acetate was used as raw material (1100°C). Scanning electron microscopy investigations emphasized the effect of the nature of barium source and synthesis conditions on the morphology of the oxide powders, as well as on the microstructure of the related ceramics.
Similar content being viewed by others
References
S Schlag HF Eicke (1994) Solid State Commun. 91 883 Occurrence Handle10.1016/0038-1098(94)90007-8 Occurrence Handle1:CAS:528:DyaK2cXms1yhurc%3D
HA Sauer JR Fisher (1960) J. Am. Ceram. Soc. 43 297 Occurrence Handle10.1111/j.1151-2916.1960.tb13657.x Occurrence Handle1:CAS:528:DyaF3cXovVelsg%3D%3D
W Heywang (1971) J. Mater. Sci. 6 1214 Occurrence Handle10.1007/BF00550094 Occurrence Handle1:CAS:528:DyaE3MXlsVGhtbc%3D
S Otta SD Bhattamisra (1994) J. Therm. Anal. Cal. 41 419 Occurrence Handle1:CAS:528:DyaK2cXjtVynurk%3D
M Stockenhuber H Mayer JA Lercher (1995) J. Am. Ceram. Soc. 76 1185 Occurrence Handle10.1111/j.1151-2916.1993.tb03738.x
HS Potdar SB Deshpande SK Date (1999) Mater. Chem. Phys. 58 121 Occurrence Handle10.1016/S0254-0584(98)00262-4 Occurrence Handle1:CAS:528:DyaK1MXhslarsrc%3D
W-S Cho (1998) J. Phys. Chem. Solids 59 659 Occurrence Handle10.1016/S0022-3697(97)00227-8 Occurrence Handle1:CAS:528:DyaK1cXjtFanurg%3D
P Durán F Capel J Tartaj C Moure (2001) J. Mater. Res. 16 197
J-D Tsay T-T Fang TA Gubiotti JY Ying (1998) J. Mater. Sci. 33 3721 Occurrence Handle10.1023/A:1004636219542 Occurrence Handle1:CAS:528:DyaK1cXmvFyit7w%3D
JO Eckert Jr CC Hung-Houston BL Gersten MM Lencka RE Riman (1996) J. Am. Ceram. Soc. 79 2929 Occurrence Handle10.1111/j.1151-2916.1996.tb08728.x
R Vivekanandan TRN Kutty (1989) Powder Technol. 57 181 Occurrence Handle10.1016/0032-5910(89)80074-9 Occurrence Handle1:CAS:528:DyaL1MXitlKisLg%3D
SW Lu BI Lee ZL Wang WD Samuels (2000) J. Cryst. Growth 219 269 Occurrence Handle10.1016/S0022-0248(00)00619-9
PP Phule SH Risbud (1988) Adv. Ceram. Mater. 3 183 Occurrence Handle1:CAS:528:DyaL1cXhsFGhsr8%3D
G Pfaff (1992) J. Mater. Chem. 2 591 Occurrence Handle10.1039/jm9920200591 Occurrence Handle1:CAS:528:DyaK38XltVarsL4%3D
Ch Lemoine B Gilbert B Michaux J-P Pirard AJ Lecloux (1994) J. Non-Cryst. Solids 175 1 Occurrence Handle10.1016/0022-3093(94)90309-3 Occurrence Handle1:CAS:528:DyaK2cXms1WlsLo%3D
C Zhixiong Z Fangaqiao L Meidong W Guoan P Xiangsheng (1991) Ferroelectrics 123 61
DP Birnie III MH Jilavi T Krajevski R Nab (1998) J. Sol-Gel Sci. Technol. 13 855 Occurrence Handle10.1023/A:1008654703057
T Yogo K-I Kikuta S Yamada S-I Hirano (1994) J. Sol-Gel Sci. Technol. 2 175 Occurrence Handle10.1007/BF00486236 Occurrence Handle1:CAS:528:DyaK2MXit1agtr4%3D
OG Dediu M Crişan Gh Aldica M Zaharescu (2004) Rev. Roum. Chim. 49 811 Occurrence Handle1:CAS:528:DC%2BD2MXisVSjtrg%3D
KS Mazdiyasni RT Dolloff JS Smith (1969) J. Am. Ceram. Soc. 52 523 Occurrence Handle10.1111/j.1151-2916.1969.tb09157.x Occurrence Handle1:CAS:528:DyaF1MXltFKjurs%3D
HP Beck W Eiser R Haberkorn (2001) J. Eur. Ceram. Soc. 21 687 Occurrence Handle10.1016/S0955-2219(00)00270-3 Occurrence Handle1:CAS:528:DC%2BD3MXislajt78%3D
JM Wilson (1995) Am. Ceram. Soc. Bull. 74 106 Occurrence Handle1:CAS:528:DyaK2MXmtFChs7g%3D
X Li W Shih (1997) J. Am. Ceram. Soc. 80 2844 Occurrence Handle10.1111/j.1151-2916.1997.tb03202.x Occurrence Handle1:CAS:528:DyaK2sXntF2jsbY%3D
S Aoyagi Y Kuroiwa A Sawada H Kawaji T Atake (2005) J. Therm. Anal. Cal. 81 627 Occurrence Handle10.1007/s10973-005-0834-z Occurrence Handle1:CAS:528:DC%2BD2MXpvVajtrk%3D
NA Pertsev AG Zembilgotov AK Tagantsev (1998) Phys. Rev. Lett. 80 1988 Occurrence Handle10.1103/PhysRevLett.80.1988 Occurrence Handle1:CAS:528:DyaK1cXhsFSnsLg%3D
M Yashima T Hoshina D Ishimura S Kobayashi W Nakamura T Tsurumi S Wada (2005) J. Appl. Phys. 98 014313-1 Occurrence Handle10.1063/1.1935132
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ianculescu, A., Brăileanu, A., Crişan, M. et al. Influence of barium source on the characteristics of sol-precipitated BaTiO3 powders and related ceramics. J Therm Anal Calorim 88, 251–260 (2007). https://doi.org/10.1007/s10973-006-8083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-8083-3