Abstract
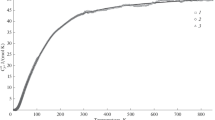
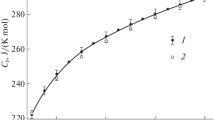
The molar heat capacities C p,m of 2,2-dimethyl-1,3-propanediol were measured in the temperature range from 78 to 410 K by means of a small sample automated adiabatic calorimeter. A solid-solid and a solid-liquid phase transitions were found at T-314.304 and 402.402 K, respectively, from the experimental C p-T curve. The molar enthalpies and entropies of these transitions were determined to be 14.78 kJ mol−1, 47.01 J K−1 mol− for the solid-solid transition and 7.518 kJ mol−1, 18.68 J K−1 mol−1 for the solid-liquid transition, respectively. The dependence of heat capacity on the temperature was fitted to the following polynomial equations with least square method. In the temperature range of 80 to 310 K, C p,m/(J K−1 mol−1)=117.72+58.8022x+3.0964x 2+6.87363x 3−13.922x 4+9.8889x 5+16.195x 6; x=[(T/K)−195]/115. In the temperature range of 325 to 395 K, C p,m/(J K−1 mol−1)=290.74+22.767x−0.6247x 2−0.8716x 3−4.0159x 4−0.2878x 5+1.7244x 6; x=[(T/K)−360]/35. The thermodynamic functions H T−H 298.15 and S T−S 298.15, were derived from the heat capacity data in the temperature range of 80 to 410 K with an interval of 5 K. The thermostability of the compound was further tested by DSC and TG measurements. The results were in agreement with those obtained by adiabatic calorimetry.
Similar content being viewed by others
References
D. K. Benson, R. W. Burrows and J. D. Webb, Solar Energy Mater., 13 (1986) 133.
J. Font, J. Muntasell, J. Navarro and J. L. Tamarit, Solar Energy Mater., 15 (1987) 403.
E. Murril and L. Breed, Thermochim. Acta, 1 (1970) 239.
V. T. Witusiewica, L. Sturz, U. Hecht and S. Rex, Acta Mater., 52 (2004) 5071.
Q. Wang, Hydrogen bonds in organic chemistry, Tianjin: Tianjin University Press, 1993.
Z. Y. Zhang, H. Zou and M. L. Yang, Gaodeng Xuexiao Huaxue Suebao, 9 (1988) 1085.
R. Kamae, K. Suenaga, T. Matsuo and H. Suga, J. Chem. Thermodyn., 33 (2001) 471.
Z.-C. Tan, G. Y. Sun and Y. Sun, J. Thermal Anal., 45 (1995) 59.
Z.-C. Tan, G. Y. Sun and Y. J. Song, Thermochim. Acta, 252–253 (2000) 247.
Z.-C. Tan, L. X. Sun and S. H. Meng, J. Chem. Thermodyn., 34 (2002) 1417.
Z.-C. Tan, B. P. Liu, J. B. Yan and L. X. Sun, Comput. Appl. Chem., 20 (2003) 264.
P. J. Gardner and K. S. Hussain, J. Chem. Thermodyn., 4 (1972) 819.
H. Saitoh, S. Ikeuchi and K. Saito, J. Therm. Anal. Cal., 81 (2005) 511.
M. H. Wang, Z.-C. Tan, Q. Shi, L.-X. Sun and T. Zhang, J. Therm. Anal. Cal., 84 (2006) 413.
S.-X. Wang, Z.-C. Tan, Q. Shi, Y. Y. Di, H. T. Zhang, F. Xu, L. X. Sun and T. Zhang, J. Chem. Thermodyn., 37 (2005) 349.
Q.-F. Tian, Z.-C. Tan, Q. Shi, F. Xu, L. X. Sun and T. Zhang, Thermochim. Acta, 430 (2005) 53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tong, B., Tan, Z.C., Lv, X.C. et al. Low-temperature heat capacities and thermodynamic properties of 2,2-dimethyl-1,3-propanediol. J Therm Anal Calorim 90, 217–221 (2007). https://doi.org/10.1007/s10973-006-7672-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7672-5