Abstract
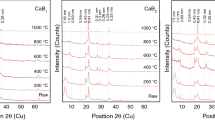
The mineralogical composition of the Kütahya calcium bentonite (CaB) from Turkey was obtained as mass% of 60% calcium rich smectite (CaS), 30% opal-CT (OCT), trace amount illite (I), and some non-clay impurities by using chemical analysis (CA), X-ray diffraction (XRD), and thermal analysis (TG-DTA) data. The crystallinity, porosity, and surface area of the samples heated between 25–1300°C for 2 h were examined by using XRD, TG, DTA and N2-adsorption-desorption data. The position of the 001 reflection which is the most characteristic for CaS does not affect from heating between 25–600°C and then disappeared. The decrease in relative intensity (I/I 0) from 1.0 to zero and the increase in full width at half-maximum peak height (FWHM) from 0.25 to 1.0° of the 001 reflection show that the crystallinity of the CaS decreased continuously by rising the heating temperature from 25 to 900°C and then collapsed. The most characteristic 101 reflection for opals intensifies greatly between 900 and 1100°C with the opal becoming more crystalline.
The total water content of the natural bentonite after dried at 25, 105 and 150°C for 48 h were determined as 8.8, 5.0 and 2.5%, respectively. The mass loss occurs between 25 and 400°C over two steps with the maximum rate at 80 and 150°C, respectively. The exact distinction of the dehydration temperatures for the adsorbed water and interlayer water is seen almost impossible. The temperature interval, maximum rate temperature, and mass loss during dehydroxylation are 400–800°C, 670°C and 4.6–5.0%, respectively. The maximum rate temperatures for decrystallization and recrystallization are 980 and 1030°C, respectively. The changes in specific micropore volume (V mi), specific mesopore volume (V me), specific surface area (S) were discussed according to the dehydration and dehydroxylation of the CaS. The V mi, V me and S reach to their maxima at around 400°C with the values of 0.045, 0.115 cm3 g−1 and 90 m2 g−1, respectively. The radii of mesopores for the bentonite heated at 400°C are distributed between 1–10 nm and intensified approximately at 1.5 nm.
Similar content being viewed by others
References
R. E. Grim, Clay Minerology, 2nd Ed., McGraw-Hill, New York 1968.
R. E. Grim and N. Güven, Bentonites, Geology, Mineralogy, Properties and Uses. Development in Sedimentology, Vol. 24, Elsevier, Amsterdam 1978.
R. M. Barrer, Clays Clay Miner., 37 (1989) 385.
E. Srasra, F. Bergaya, H. van Damme and N. K. Ariquib, Appl. Clay Sci., 4 (1989) 411.
E. Gamiz, J. Linares and R. Delgado, Appl. Clay Sci., 6 (1992) 359.
D. M. Moore and R. C. Reynolds Jr., X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd Ed., Oxford University Press, Oxford 1997.
H. H. Murray, Appl. Clay Sci., 17 (2000) 207.
T. J. Pinnavaia, Science, 220 (1983) 365.
R. S. Varma, Tetrahedron, 58 (2002) 1235.
M. C. Wang, J. M. Benway and A. M. Arayssi, In Physicochemical Aspects of Soil and Related Materials, K. B. Hoodinott, R. O. Lamb and A. J. Lutenegger, Eds, ASTM STP 1095, Philadelphia 1990, pp. 1139–1158.
M. M. Abu-Zreig, N. M. Al-Akhras and M. F. Attom, Appl. Clay Sci., 20 (2001) 129.
S. Chandrasekhar and S. Ramaswamy, Appl. Clay Sci., 21 (2002) 133.
Ö. Tan, L. Yılmaz and S. Zamioğlu, Mater. Lett., 58 (2004) 1176.
I. Kolaríková, R. Prikryl, R. Hanus and E. Jelínek, Appl. Clay Sci., 29 (2005) 215.
W. F. Bradley and R. E. Grim, Am. Mineral., 36 (1951) 182.
G. W. Brindley, Ceramica, 24 (1978) 217.
T. Mozas, S. Bruque and A. Rodriquez, Clay Miner., 15 (1980) 421.
W. T. Reicle, J. Catal., 94 (1985) 547.
H. Ceylan, A. Yıldız and Y. Sarıkaya, Turk. J. Chem., 17 (1993) 267.
R. C. Joshi, G. Achari, D. Horfield and T. S. Nagaraj, J. Geotech. Eng. ASCE, 120 (1994) 1080.
M. Chorom and P. Rengasamy, Clays Clay Miner., 44 (1996) 783.
A. Neaman, M. Pelletier and F. Willieras, Appl. Clay Sci., 22 (2003) 153.
V. Balek, Z. Malék, S. Yariv and G. Matuschek, J. Therm. Anal. Cal., 56 (1999) 67.
E. Kristóf-Makó and A. Z. Juhász, Thermochim. Acta, 342 (1999) 105.
M. V. Kök and W. Smykatz-Kloss, J. Therm. Anal. Cal., 64 (2001) 1271.
V. Hlavatý and V. S. Fajnor, J. Therm. Anal. Cal., 67 (2002) 113.
M. V. Kök, Energy Sources, 24 (2002) 899.
M. V. Kök, Energy Sources, 26 (2004) 145.
S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, 2nd Ed., Academic Press, London 1982.
J. M. Adams, Appl. Clay Sci., 2 (1987) 309.
Z. Ge, D. Li and T. J. Pinnavaia, Microporous Mater., 3 (1994) 165.
P. Kumar, R. V. Jasra and T. S. G. Bhat, Ind. Eng. Chem. Res., 34 (1995) 1440.
D. R. Brown and C. N. Rhodes, Catal. Lett., 45 (1997a) 35.
M. Önal, Y. Sarıkaya, T. Alemdaroğlu and İ. Bozdoğan, Turk. J. Chem., 27 (2003) 683.
Y. Sarıkaya, M. Önal, B. Baran and T. Alemdaroğlu, Clays Clay Miner., 48 (2000) 557.
T. Alemdaroğlu, G. Akkuş, M. Önal and Y. Sarıkaya, Turk. J. Chem., 27 (2003) 675.
H. Noyan, M. Önal and Y. Sarıkaya, Clays Clay Miner., 54 (2006) 377.
N. Yıldız, Y. Sarıkaya and A. Çalımlı, Appl. Clay Sci., 14 (1999) 319.
M. Önal, Y. Sarıkaya, T. Alemdaroğlu and İ. Bozdoğan, Turk. J. Chem., 26 (2002) 409.
N. A. Talvitie, Anal. Chem., 23 (1951) 623.
J. M. Elzea, J. E. Odom and W. J. Miles, Anal. Chim. Acta, 286 (1994) 107.
S. Kahraman, M. Önal, Y. Sarıkaya and İ. Bozdoğan, Anal. Chim. Acta, 552 (2005) 201.
Y. Sarıkaya and S. Aybar, Commun. Fac. Sci. Uni. Ank., 24B (1978) 33.
Y. Sarıkaya, İ. Sevinç and M. Akinç, Powder Technol., 116 (2001) 109.
M. Gal, J. Thermal Anal., 37 (1991) 1621.
A. Acosta, I. Iglesias, M. Aineto, M. Romero and J. Ma. Rincón, J. Therm. Anal. Cal., 67 (2002) 249.
H. Zou, M. Li, J. Shen and A. Auroux, J. Therm. Anal. Cal., 72 (2003) 209.
A. Fodor, L. Ghizdavu, A. Suteu and A. Caraban, J. Therm. Anal. Cal., 75 (2004) 153.
J. Ma. Rincón, M. Romero, A. Hidalgo and Ma. J. Liso, J. Therm. Anal. Cal., 76 (2004) 903.
N. Yener, M. Önal, G. Üstünışık and Y. Sarıkaya, J. Therm. Anal. Cal., OnlineFirst, DOI: 10.1007/s10973-005-7459-0.
H. Bayram, M. Önal, G. Üstünışık and Y. Sarıkaya, J. Therm. Anal. Cal., OnlineFirst, DOI: 10.1007/s10973-006-7561-y.
S. Brunauer, L. S. Deming, D. M. Deming and E. Teller, J. Am. Chem. Soc., 62 (1940) 1723.
F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by Powder and Porous Solids, Academic Press, London 1999.
B. G. Linsen, Physical and Chemical Aspects of Adsorbent and Catalysts, Academic Press, London 1970.
S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 60 (1938) 308.
A. L. McClellan and H. F. Hornsberger, J. Colloid Interface Sci., 23 (1967) 577.
D. H. Everett, G. D. Parfitt, K. S. W. Sing and R. Wilson, J. Appl. Chem. Biotechnol., 24 (1974) 199.
A. U. Doğan, M. Doğan, M. Önal, Y. Sarıkaya, A. Aburub and D. E. Wurster, Clays Clay Miner., 54 (2006) 62.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Önal, M., Sarıkaya, Y. Thermal behavior of a bentonite. J Therm Anal Calorim 90, 167–172 (2007). https://doi.org/10.1007/s10973-005-7799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7799-9