Abstract
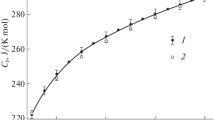
Low-temperature heat capacities of a solid complex Zn(Val)SO4·H2O(s) were measured by a precision automated adiabatic calorimeter over the temperature range between 78 and 373 K. The initial dehydration temperature of the coordination compound was determined to be, T D=327.05 K, by analysis of the heat-capacity curve. The experimental values of molar heat capacities were fitted to a polynomial equation of heat capacities (C p,m) with the reduced temperatures (x), [x=f (T)], by least square method. The polynomial fitted values of the molar heat capacities and fundamental thermodynamic functions of the complex relative to the standard reference temperature 298.15 K were given with the interval of 5 K.
Enthalpies of dissolution of the [ZnSO4·7H2O(s)+Val(s)] (Δsol H m,l 0) and the Zn(Val)SO4·H2O(s) (Δsol H m,2 0) in 100.00 mL of 2 mol dm–3 HCl(aq) at T=298.15 K were determined to be, Δsol H m,l 0=(94.588±0.025) kJ mol–1 and Δsol H m,2 0=–(46.118±0.055) kJ mol–1, by means of a homemade isoperibol solution–reaction calorimeter. The standard molar enthalpy of formation of the compound was determined as: Δf H m 0 (Zn(Val)SO4·H2O(s), 298.15 K)=–(1850.97±1.92) kJ mol–1, from the enthalpies of dissolution and other auxiliary thermodynamic data through a Hess thermochemical cycle. Furthermore, the reliability of the Hess thermochemical cycle was verified by comparing UV/Vis spectra and the refractive indexes of solution A (from dissolution of the [ZnSO4·7H2O(s)+Val(s)] mixture in 2 mol dm–3 hydrochloric acid) and solution A’ (from dissolution of the complex Zn(Val)SO4·H2O(s) in 2 mol dm–3 hydrochloric acid).
Similar content being viewed by others
References
M. Mahmoud, S. Abdel-Monem and M. Paul, U.S. Pat. US 4 039 681, 1977-08-02, Chem. Abstr. 1977, 87, 15196.
S. Taguchi,M. Inokuchi, N. Nakajima, M. Inomata and Y. Natitoh, WO Pat. 10 178, 1992-06-25, Chem. Abstr. 1992, 117, 258218.
H. Harvey and U. K. Ashmed, U.S. Pat. US 4 830 716, 1989-05-16, Chem. Abstr. 1989, 110, 219070.
XY Zhang XW Yang Y Jia SL Gao (2000) Chin. J. Appl. Chem. 17 850-854
HY Jiang DH Ren HF Xie (1986) Chin. J. Northwest Uni. (Natural Science Edition) 22 1
ZC Tan B Xue SW Lu SH Meng XH Yuan YJ Song (2001) J. Therm. Anal. Cal. 63 297 Occurrence Handle10.1023/A:1010121427777 Occurrence Handle1:CAS:528:DC%2BD3MXhs1Sjur4%3D
S X.Wang ZC Tan YY Di F Xu MH Wang LX Sun T Zhang (2004) J. Therm. Anal. Cal. 76 335 Occurrence Handle10.1023/B:JTAN.0000027833.24442.a0
DA Ditmars S Ishihara SS Chang G Bernstein ED West (1982) J. Res. Natl. Bur. Stand. 87 159 Occurrence Handle1:CAS:528:DyaL38XltFKht74%3D
YY Di ZC Tan XH Sun MH Wang F Xu YF Liu LX Sun HT Zhang (2004) J. Chem. Thermodyn. 36 79 Occurrence Handle10.1016/j.jct.2003.08.017 Occurrence Handle1:CAS:528:DC%2BD2cXmtFegsA%3D%3D
YY Di ZC Tan SL Gao SX Wang (2004) J. Chem. Eng. Data 49 965 Occurrence Handle10.1021/je034264n Occurrence Handle1:CAS:528:DC%2BD2cXktlGkt7k%3D
D D.Wagman WH Evans VB Parker RH Schumm I Halow SM Bailey KL Churney RL Nuttall (1982) Phys. Chem. Ref. Data 11 (suppl. 2) 38, 140
JO Hutchens AG Cole JW Stout (1963) J. Phys. Chem. 67 1128 Occurrence Handle10.1021/j100799a047 Occurrence Handle1:CAS:528:DyaF3sXnslaktA%3D%3D
JA Dean et al. (1991) Lange’s Handbook of Chemistry, 13th Ed. Sci. Press Beijing 1986
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di, Y.Y., Tan, Z.C., Li, L.W. et al. Low-temperature heat capacities and standard molar enthalpy of formation of the complex. J Therm Anal Calorim 87, 545–551 (2007). https://doi.org/10.1007/s10973-005-7431-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7431-z