Abstract
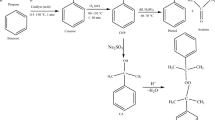
Cumene hydroperoxide (CHP) is classified as a flammable hazard in NFPA 43B. Fires or explosions induced by thermal hazards ascribed to the unstable hydroperoxyl or peroxyl groups are often reported. This sequence studies is aimed at the decomposition phenomena associated with the reactive and incompatible characteristics of CHP mixed with alkaline solutions. Various alkalines were used for comparing the relative impact of bases and effects on concentrations. Exothermic onset temperatures and heats of decomposition of these incompatible mixtures were performed by differential scanning calorimetry (DSC). Comparisons of exothermic onset temperature, peak power, heat of decomposition, etc., were assessed to verify the severity of incompatible hazards in these systems. When mixed with a small amount of the hydroxides (in the production or storage of CHP), CHP will be more labile or unstable because of lower exothermic temperature. In addition, to elucidate the final products and propose mechanisms of the reaction of CHP mixed with alkaline solution, the analytical results were carried out by GC/MS and IR. The exhibited reactivity was complicated and significantly affected by the alkaline solutions. The reaction schemes have been proposed in this study. These results are especially important in process safety design for producing CHP and its related compounds, such as phenol, α-cumyl alcohol (CA), acetophenone (AP), and dicumyl peroxide (DCPO).
Similar content being viewed by others
References
PY Yeh CM Shu YS Duh (2003) Ind. Eng. Chem. Res. 42 1 Occurrence Handle1:CAS:528:DC%2BD38Xpt1yntL8%3D Occurrence Handle10.1021/ie011035q
TC Ho YS Duh JR Chen (1998) AIChE Loss Prevention Symposium Paper 6F 1
YS Duh CS Kao HH Hwang WW-L Lee (1998) Trans. IChemE 76B 271
HY Hou CM Shu YS Duh (2001) AIChE J. 47 1893 Occurrence Handle1:CAS:528:DC%2BD3MXlvFKqtL0%3D Occurrence Handle10.1002/aic.690470819
YW Wang CM Shu YS Duh CS Kao (2001) Ind. Eng. Chem. Res. 40 1125 Occurrence Handle1:CAS:528:DC%2BD3MXhtVyisLk%3D Occurrence Handle10.1021/ie990900s
HY Hou TS Liao YS Duh CM Shu (2006) J. Therm. Anal. Cal. 83 167 Occurrence Handle1:CAS:528:DC%2BD28XitF2nt70%3D Occurrence Handle10.1007/s10973-005-7054-4
ME Levin NO Gonzales LW Zimmerman J Yang (2006) J. Hazard. Mater. 130 88 Occurrence Handle1:CAS:528:DC%2BD28XitVSjsLs%3D Occurrence Handle10.1016/j.jhazmat.2005.07.068
D. E. Clark, Chem. Heal. Saf., September/October 2001.
TA Kletz (1988) Plant/Oper. Prog. 7 226 Occurrence Handle1:CAS:528:DyaL1MXksVSjug%3D%3D Occurrence Handle10.1002/prsb.720070406
InstitutionalAuthorNameNational Fire Protection Association et al. (2002) National Fire Protection Association Code for the Storage of Organic Peroxide Formulations, NFPA 43B Minneapolis, MN, USA
U. N. Recommendations on the Transport of Dangerous Goods, United Nations Publications: Geneva, Switzerland, 13rd Edition, 1 (2003) 104.
InstitutionalAuthorNameMettler Company et al. (1998) TA8000 Operation Instructions Mettler Company Switzerland
SS Shashin ON Emanuel (1987) Izv. Akad. Nauk SSSr, Ser. Khim. 1 534
AS Lyavinets (2000) Russian J. Phys. Chem. 74 1072
R Hiatt J Clipcham T Visser (1964) Can. J. Chem. 42 2754 Occurrence Handle1:CAS:528:DyaF2MXivFeitA%3D%3D Occurrence Handle10.1139/v64-408
R Hiatt T Mill C Irwin JK Castelman (1968) J. Org. Chem. 33 1421 Occurrence Handle1:CAS:528:DyaF1cXhtVChtbw%3D Occurrence Handle10.1021/jo01268a023
MS Kharasch A Fono W Nudenberg (1950) J. Org. Chem. 15 113
JJ Zwolenik (1966) J. Phys. Chem. 8 2464
AA Aldeeb WJ Rogers MS Mannan (2003) J. Hazard. Mater. 104 269 Occurrence Handle1:CAS:528:DC%2BD3sXosFOqsL8%3D Occurrence Handle10.1016/S0304-3894(03)00269-3
GJ Suppes MA McHugh (1989) Ind. Eng. Chem. Res. 28 1146 Occurrence Handle1:CAS:528:DyaL1MXksF2qt78%3D Occurrence Handle10.1021/ie00092a005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, H.Y., Duh, Y.S., Lin, W.H. et al. Reactive incompatibility of cumene hydroperoxide mixedwith alkaline solutions. J Therm Anal Calorim 85, 145–150 (2006). https://doi.org/10.1007/s10973-005-7364-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7364-6