Abstract
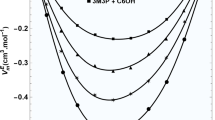
The molar heat capacities of the binary mixture composed of water and n-butanol were measured with an adiabatic calorimeter in the temperature range 78–320 K. The functions of the heat capacity with respect to thermodynamic temperature were established. A glass transition, solid–solid phase transition and solid–liquid phase transition were observed. The corresponding enthalpy and entropy of the solid–liquid phase transition were calculated, respectively. The thermodynamic functions relative to a temperature of 298.15 K were derived based on the relationships of the thermodynamic functions and the function of the measured heat capacity with respect to temperature.
Similar content being viewed by others
References
Z Nan ZC Tan LX Sun (2004) Acta Phys. Chim. Sin. 20 626 Occurrence Handle1:CAS:528:DC%2BD2cXmvVCjtb8%3D
Z Nan ZC Tan (2005) J. Chem. Eng. Data 50 6 Occurrence Handle10.1021/je0342668 Occurrence Handle1:CAS:528:DC%2BD2cXhtVejtr7J
R Páramo M Zouine C Casanova (2002) J. Chem. Eng. Data 47 441 Occurrence Handle10.1021/je0155103
Z Nan ZC Tan (2004) J. Therm. Anal. Cal. 76 955 Occurrence Handle10.1023/B:JTAN.0000032281.40952.7e Occurrence Handle1:CAS:528:DC%2BD2cXkvFGgt78%3D
SX Wang ZC Tan YY Di F Xue MH Wang LX Sun T Zhang (2004) J. Therm. Anal. Cal. 76 335 Occurrence Handle10.1023/B:JTAN.0000027833.24442.a0 Occurrence Handle1:CAS:528:DC%2BD2cXjvFSrs7g%3D
YJ Song ZC Tan SW Lu Y Xue (2004) J. Therm. Anal. Cal. 77 873 Occurrence Handle10.1023/B:JTAN.0000041666.78862.90 Occurrence Handle1:CAS:528:DC%2BD2cXnsFOktrs%3D
P Xue JY Wang ZC Tan SW Lu SH Meng (2004) J. Therm. Anal. Cal. 76 965 Occurrence Handle10.1023/B:JTAN.0000032282.04071.f1 Occurrence Handle1:CAS:528:DC%2BD2cXkvFGgtrY%3D
BP Liu ZC Tan ZD Nan P Liu LX Sun F Xue XZ Lan (2003) J. Therm. Anal. Cal. 71 623 Occurrence Handle10.1023/A:1022824530862 Occurrence Handle1:CAS:528:DC%2BD3sXitVynsL0%3D
F Xu LX Sun ZC Tan P Yu T Zhang (2003) J. Therm. Anal. Cal. 74 335 Occurrence Handle10.1023/A:1026366928276 Occurrence Handle1:CAS:528:DC%2BD3sXosVGgt70%3D
JM Sørensen W Arlt et al. (1979) Chemistry Data Series Dechema Frankfurt, Germany Vol. V, part 1.
K Tamura J Hu C Trandum P Westh CA Haynes Y Koga (2000) Phys. Chem. Chem. Phys. 2 355 Occurrence Handle10.1039/a908422c Occurrence Handle1:CAS:528:DC%2BD3cXlvVOisA%3D%3D
H Ogawa S Murakami (1986) Thermochim. Acta 109 145 Occurrence Handle10.1016/0040-6031(86)85016-X Occurrence Handle1:CAS:528:DyaL2sXptFajug%3D%3D
DG Archer (1993) J. Phys. Chem. Ref. Data 22 1441 Occurrence Handle10.1063/1.555931 Occurrence Handle1:CAS:528:DyaK2cXmtlGqsg%3D%3D
GS Parks (1925) J. Am. Chem. Soc. 47 338 Occurrence Handle10.1021/ja01679a009 Occurrence Handle1:CAS:528:DyaB2MXotVWk
E Zorebski M Chroazewski M Traczyk (2005) J. Chem. Thermodyn. 37 281 Occurrence Handle10.1016/j.jct.2004.09.007 Occurrence Handle1:CAS:528:DC%2BD2cXhtFaqu7fF
ZH Liu T Hatakeyama et al. (2000) Handbook of Analytical Chemistry 2nd Chemical Industry Press Beijing 64
JF Masson GM Polomark (2002) Energy Fuels 16 470 Occurrence Handle10.1021/ef010233r Occurrence Handle1:CAS:528:DC%2BD38Xps1Wqug%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nan, Z., Tan, ZC. Thermodynamic properties of the binary mixture of water and n-butanol. J Therm Anal Calorim 87, 539–544 (2007). https://doi.org/10.1007/s10973-005-7295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7295-2