Abstract
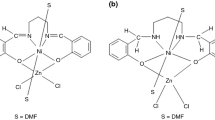
The complexes of the type SnCl4(HL)·EtOH and SnCl2L2 (HL 1 : the Schiff base resulted in 1:1 condensation of isatin and aniline; HL 2 : the Schiff base resulted in 1:1 condensation of isatin and p-toluidine) have been synthesized and characterized. The thermal analysis of the new ligands and complexes has evidenced the thermal intervals of stability and also the thermal effects that accompany them. The Schiff bases thermal transformations consist in phase transitions, Carom–N bond cleavage and thermolysis processes. The different nature of the complexes generates their different thermal behaviour. The complexes lead in three steps to SnO2 and in all cases the Schiff bases degradation generates a pyrrolidone-coordinated derivative. As for the SnCl4(HL)·EtOH complexes, the SnCl4 formed during the last step is involved in two competitive processes, one consists in their volatilisation while the other one leads to SnO2. As result the SnO2 residue is smaller than the theoretically expected.
Similar content being viewed by others
References
M Verma SN Pandeya KN Singh JP Stables (2004) Acta Pharm. 54 49 Occurrence Handle1:CAS:528:DC%2BD2cXjtVamtbc%3D
JFM da Silva SJ Garden AC Pinto (2001) J. Braz. Chem. Soc. 67 273
J. M. Law, W. Henderson and B. K. Nicholson, J. Chem. Soc., Dalton Trans., (1997) 4587.
VI Thapcov NM Samucy (1996) J. Obsch. Khim. 66 1692
A Lenz K Sünkel W Beck (1996) Z. Naturforsch. 51b 1639
AM Hassaan MA Khalifa AK Shehata (1995) Bull. Soc. Chim. Belg. 104 121 Occurrence Handle1:CAS:528:DyaK2MXltFSksrc%3D Occurrence Handle10.1002/bscb.19951040302
RM El Bahnasawy E El Shereafy TI Kashar (1994) Egypt. J. Chem. 37 333 Occurrence Handle1:CAS:528:DyaK2MXmtFWltLk%3D
K Anil S Gupta AG Gupta (1983) Eur. J. Med. Chem. 18 181
ZH Chohan H Pervez A Rauf KM Khan CT Supuran (2004) J. Enz. Inhib. Med. Chem. 19 417 Occurrence Handle1:CAS:528:DC%2BD2cXhtFSnsbfK Occurrence Handle10.1080/14756360410001710383
NK Singh N Agarwal RC Agarwal (1985) Ind. J. Chem. 24A 617 Occurrence Handle1:CAS:528:DyaL2MXmtVCktL0%3D
V. Y. Kukushkin, T. Nishioka, S. Nakamura, I. Kinoshita and K. Isobe, Chem. Lett., (1977) 189.
A Hudák A Košturiak (1999) J. Therm. Anal. Cal. 58 579 Occurrence Handle10.1023/A:1010148310379
A Kriza C Parnau (2001) Acta Chim. Slov. 48 445 Occurrence Handle1:CAS:528:DC%2BD3MXntFWhtLc%3D
CA Ribeiro MS Crespi LS Guinesi CTR Guerreiro HE Zorel (2001) J. Therm. Anal. Cal. 64 1209 Occurrence Handle1:CAS:528:DC%2BD3MXlslKqurY%3D Occurrence Handle10.1023/A:1011557431951
RS Barbieri AKC Dias SF da Silva VR Terra EP de Lima (2005) J. Therm. Anal. Cal. 79 255 Occurrence Handle1:CAS:528:DC%2BD2MXkt1Ciu74%3D Occurrence Handle10.1007/s10973-005-0044-8
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parnau, C., Olar, R., Badea, M. et al. Thermal behavior of some new isatin complexes. J Therm Anal Calorim 86, 217–221 (2006). https://doi.org/10.1007/s10973-005-7178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7178-6