Abstract
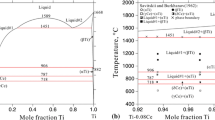
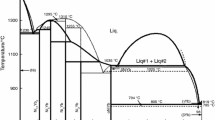
The thermodynamic exploitation of the solid–liquid equilibria in the MIPO3–Pb(PO3)2, MIPO3–Cu(PO3)2 and MIPO3–Ce(PO3)3 systems (with M I=Li, Na, K, Rb, Cs, Ag, Tl) is carried out using a semi-empirical equation of the liquidus curves already used with success for similar binary systems.
The enthalpy of fusion is calculated for each pure polyphosphate on the assumption that the liquid solution is ideal and only formed by MIPO3 and M(PO3)q entities (q=2 for Pb and Cu, q=3 for M=Ce). In the most binary systems, a wide difference between the calculated values of the melting enthalpies of these polyphosphates and the measured ones determined from the DTA curves, was observed. This difference is probably due to the existence of some molecular associations in the liquid phase.
The enthalpy of fusion of each terminal phase was then recalculated on the assumption that the liquid contains a molecular association of the type of MI pnMn(PO3)n(q+p) in the region of the diagram rich in MIPO3 or a molecular association of the type of MI nMnp(PO3)n(qp+1) in the region rich in M(PO3)q (q=2 for Pb and Cu, q=3 for M=Ce). In this case, the obtained values are in good agreement with experimental determinations.
Similar content being viewed by others
References
JC Grenier I Mahama (1972) C. R. Acad. Sci. 274C 1063
K Von H Jost (1964) Acta Cryst. 17 1539 Occurrence Handle10.1107/S0365110X64003838
JC Guitel I Tordjman (1976) Acta Cryst. B 32 2960
M Laügt (1969) C. R. Acad. Sci. 269C 1122
M Laügt M Scory A Durif (1968) Mater. Res. Bull. 3 963 Occurrence Handle10.1016/0025-5408(68)90108-6
M Laügt A Durif C Martin (1968) C. R. Acad. Sci. 266C 1700
M Laügt JC Guitel A Durif C Martin (1967) C. R. Acad. Sci. 265C 741
M Laügt (1968) C. R. Acad. Sci. 267C 1489
M Laügt C Martin (1972) Mater. Res. Bull. 7 1525 Occurrence Handle10.1016/0025-5408(72)90190-0
M Rzaigui et al. (1983) Thèse de doctorat Faculté des Sciences de Tunis Tunisie
C Marhag D Ben Hassen-Chehimi H Said (2003) Thermochim. Acta 397 55 Occurrence Handle1:CAS:528:DC%2BD3sXltlSrug%3D%3D Occurrence Handle10.1016/S0040-6031(02)00315-5
C Marhag D Ben Hassen-Chehimi C Favotto H Said (2003) Phys. Chem. News 12 11
C Marhag D Ben Hassen-Chehimi H Said (2004) J. Therm. Anal. Cal. 76 417 Occurrence Handle1:CAS:528:DC%2BD2cXktVWhurk%3D Occurrence Handle10.1023/B:JTAN.0000028021.41599.71
JJ Counioux R Tenu (1981) J. Chim. Phys. 78 815, 823
JJ Counioux R Tenu (1985) J. Chim. Phys. 82 43 Occurrence Handle1:CAS:528:DyaL2MXktFaisLY%3D
D Ben Hassen-Chehimi N Kbir Ariguib M Trabelsi R Tenu JJ Counioux (1987) Thermochim. Acta 116 85 Occurrence Handle10.1016/0040-6031(87)88168-6
A Durif et al. (1995) Crystal Chemistry of Condensed Phosphates Plenum Press New York and London
J. Saurel, J. Debaene and J. J. Baron, Techniques de l’Ingénieur (constants), Paris, K2 (1989) K600.
D Ben Hassen-Chehimi et al. (1983) Thèse de doctorat Faculté des Sciences de Tunis Tunisie
C Marhag H Said P Satre C Favotto J Rogez (2003) J. Therm. Anal. Cal. 74 275 Occurrence Handle1:CAS:528:DC%2BD3sXosVGgtrk%3D Occurrence Handle10.1023/A:1026354525550
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marhag, C., Ben Hassen-Chehimi, D. & Said, H. Thermodynamic exploitation of the liquidus curves in the MIPO3–Pb(PO3)2, MIPO3–Cu(PO3)2 and MIPO3–Ce(PO3)3 systems (M I=Li, Na, K, Rb, Cs, Ag, Tl). J Therm Anal Calorim 86, 249–254 (2006). https://doi.org/10.1007/s10973-005-7135-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7135-4