Abstract
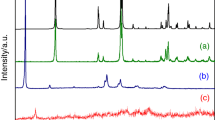
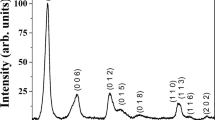
Transition metal containing hafnium phosphates forms has layered monoclinic structure. In general these materials have similar route of thermal decomposition; i.e. they loss their crystal water first then at a higher temperature their structural one. At least the result HfP2O7 goes through phase change at about 1000 K. In detail among their thermal decomposition some differences occur. The Mn and Zn containing samples have similar behaviour as pure hafnium phosphate. The Cu and Ni containing materials have an additional exo-process connected with the transition metal oxide forms. In case of Co containing sample similar to that of Zn containing one (but very weak) processes were observed.
Similar content being viewed by others
References
Y. Elmismari, A. Dehair, L. Szirtes and S. K. Shakshooki, J. Radioanal. Nucl. Chem. Art., 158 (1992) 3.
S. K. Shakshooki, N. Naqvi, J. Kowalczyk, S. Khalil, M. Rais and F. Tarish, React. Polym., 7 (1988) 221.
S. K. Shakshooki, N. Naqvi, S. Khalil, M. Mostaq and L. Szirtes, J. Radioanal. Nucl. Chem. Art., 121 (1988) 195.
S. K. Shakshooki, F. Masaodi, A Dehair, L. Szirtes and J. Kowalczyk, J. Radioanal. Nucl. Chem. Art., 132 (1989) 251.
G. C. Hadjipanayis and R. W. Siegel (Eds), Nanophase Materials: Synthesis, Properties, Applications, Kluwer, Dordrecht, 1994 and references herein.
K. Mészáros-Szécsényi, V. M. Leovac, Z. K. Jacimovic and G. Pokol, J. Therm. Anal. Cal., 74 (2003) 943.
V. Logvinenko, L. Yudanova, N. Yudanov and G. Chekhova, J. Therm. Anal. Cal., 74 (2003) 395.
L. Szirtes, J. Megyeri, L. Riess and E. Kuzmann, J. Therm. Anal. Cal., 65 (2001) 975.
L. Szirtes, J. Megyeri and E. Kuzmann, Solid State Ionics in press.
E. B. Sandell (ed.), Colorimetric Determination of Traces of Metals, Intersci. Publ. Inc. N.Y. 1959.
E. Schulek and Z. Szabó, Theory and methods of quantitative analysis, Tankönyvkiadó, Budapest 1971, p. 418 (in Hungarian).
L. Szirtes, J. Megyeri, L. Riess and E. Kuzmann, J. Therm. Anal. Cal., 63 (2001) 117.
S. K. Shakshooki, A. Dehair, L. Szirtes and Yu.V. Yakovlev, J. Radioanal. Nucl. Chem. Lett., 154 (1991) 23.
L. Szirtes, J. Megyeri and E. Kuzmann, J. Crystallogr. to be published.
L. Szirtes, J. Megyeri, E. Kuzmann and Z. Klencsár, Solid State Ionics, 145 (2001) 257.
L. Szirtes, L. Riess and J. Megyeri, J. Therm. Anal. Cal., 73 (2003) 209.
S. Viano, R. Mishra, J. Lloyd, T. Losby and T. Gheyi, J. Non-Cryst. Solids, 325 (2003) IBNLFASSI16.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szirtes, L., Riess, L. & Megyeri, J. Thermoanalytical investigation of crystalline layered hafnium salts. J Therm Anal Calorim 79, 135–140 (2005). https://doi.org/10.1007/s10973-004-0574-5
Issue Date:
DOI: https://doi.org/10.1007/s10973-004-0574-5