Abstract
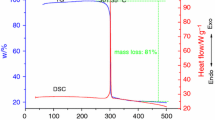
The heat characteristics of sulfur mustard (H), Lewisite (L), LI, LII, LIII, yellow agent (1:1 mass/mass H and L) and H heels were investigated by thermogravimetric - differential thermal analysis (TG-DTA). The object of this study was to provide details of the effectiveness of elevated temperature on the decomposition of these agents in both an active atmosphere (air) and an inert atmosphere (nitrogen). TG-DTA measured object mass change and heat radiation/absorption corresponding to regulated temperature changes of the test materials. All agents, with the exception of one of the H heels, exhibited an endotherm suggesting evaporation of the agents rather than oxidation.
Similar content being viewed by others
References
R. G. Manley, Pure Appl. Chem., 74 (2002) 2235.
G. S. Pearson and R. S. Magee, Pure Appl. Chem., 74 (2002) 187.
G. P. Glasby, Sci. Total Environ., 206 (1997) 267.
A. M. Boronin, I. Termakova, V. G. Sakharovsky, G. M. Grechkina, I. I. Starovoitov, R. L. Autenrieth and J. R. Wild, J. Chem. Technol. Biotechnol., 75 (2000) 82.
N. B. Munro, S. S. Talmage, G. D. Griffin, L. C. Waters, A. P. Watson, J. F. King and V. Hauschild, Environ. Health Perspect., 107 (1999) 933.
J. Henrikson, A. Johannisson, P. E. Bergqvist and L. Norrgren, Arch. Environ. Contam. Toxicol., 30 (1996) 213.
D. B. Ludlum and B. Papirmeister, Basic Life Sci., 38 (1986) 119.
A. P. Watson and G. D. Griffin, Environ. Health Perspect., 98 (1992) 259.
W. P. Bozeman, D. Dilbero and J. L. Schauben, Emerg. Med. Clin. N. Am., 20 (2002) 975.
N. B. Munro, A. P. Watson, K. R. Ambrose and G. D. Griffin, Environ. Health Perspect., 89 (1990) 205.
S. Carnes, Environ. Prof., 11 (1989) 279.
D. K. Rohrbaugh and Y. Yang, J. Mass Spectrom., 32 (1997) 1247.
G. W. Wagner, B. K. Maciver, D. K. Rohbaugh and Y. Yang, Phosphorus, Sulfur and Silicon, 152 (1999) 65.
P. S. Thomas, D. Hirschausen, R. E. White, J. P. Guerbois and A. S. Ray, J. Therm. Anal. Cal., 72 (2003) 769.
N. Sergent, P. Gélin, L. Périer-Camby, H. Praliaud and G. Thomas, J. Therm. Anal. Cal., 72 (2003) 1117.
S. Arvelakis, F. J. Frandsen and K. Dam-Johansen, J. Therm. Anal. Cal., 72 (2003) 1005.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carrick, W., Fernee, L. & Francis, D. Heat characteristics of chemical warfare agents. J Therm Anal Calorim 79, 101–106 (2005). https://doi.org/10.1007/s10973-004-0569-2
Issue Date:
DOI: https://doi.org/10.1007/s10973-004-0569-2