Abstract
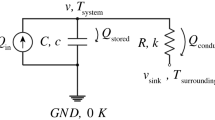
The non-linear least squares fitting of the chemical heat flow and the reactor temperature are compared for reactions with pseudo-first order chemical kinetics carried out in an isoperibolic calorimeter operating quasi-isothermally. Both methods give very similar results for the reaction rate constant and enthalpy of reaction but fitting the reactor temperature appears to have some advantages especially when there is an enthalpy of mixing of the reagents.
Similar content being viewed by others
References
J. Rouquerol and W. Zielenkiewicz, Thermochim. Acta, 109 (1986) 121.
J. P. Jackobsen, Thermochim. Acta, 160 (1990) 13.
P. W. Atkins, Physical Chemistry 6th Ed., Oxford University Press, Oxford 1998, Chapter 25.
W. Regenass, Thermochim. Acta, 95 (1985) 351.
L. G. Karlsen and J. Villadsen, Chem. Eng. Sci., 42 (1987) 1165.
V. Gold, Trans. Faraday Soc., 44 (1948) 506.
J. March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, McGraw-Hill, New York 1968, p. 308.
A. Tian, Bull. Soc. Chim. Fr., 33 (1923) 427.
R. Nomen, J. Sempere, K. Aviles and F. Pieper, J. Therm. Anal. Cal., 72 (2003) 991.
A. Savitzky and M. J. E. Golay, Anal. Chem., 38 (1964) 1627.
M. L. Boas, Mathematical Methods in the Physical Sciences, Wiley 1983, Chap. 8.
G. De Domenico, D. G. Lister, G. Maschio and A. Stassi, J. Therm. Anal. Cal., 66 (2001) 815.
C. Ampelli, D. Di Bella, D. G. Lister, G. Maschio and J. Parisi, J. Therm. Anal. Cal., 72 (2003) 875.
D. W. Marquardt, J. Soc. Ind. Appl. Math., 11 (1963) 431.
∑Plot, version 8.0, SPSS Inc., Chicago 2002.
D. R. Lide and H. V. Kehiaian, CRC Handbook of Thermophysical and Thermochemical Data, CRC Press LLC, Bocca Raton 1994, p. 125.
R. C. Reid, J. M. Prausnitz and B. E. Poling, The Properties of Gases and Liquids, McGraw-Hill, New York 1988, p. 137.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ampelli, C., Di Bella, D., Lister, D.G. et al. Fitting isoperibolic calorimeter data for reactions with pseudo-first order chemical kinetics. J Therm Anal Calorim 79, 89–94 (2005). https://doi.org/10.1007/s10973-004-0567-4
Issue Date:
DOI: https://doi.org/10.1007/s10973-004-0567-4