Abstract
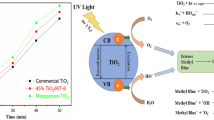
A sol-gel method was applied to prepare the porous ytterbium-doped potassium titanate. The materials were obtained by changing the chain length of polyethylene glycol (PEG) in the precursor. The PEG in the sol might be beneficial for the growth of K2Ti6O13 crystals, but the PEG did not lead to any phase transformation. The pores in the Yb-K2Ti6O13(n) samples became larger with a rising PEG chain length, and the particles also became larger. The largest BET surface area (26.79 m2/g) and pore volume (0.0465 cm3/g) were obtained for the Yb-K2Ti6O13(2000). The largest number of hydroxyl radicals were generated by the Yb-K2Ti6O13(2000) that had the strongest photocatalytic activity, and a total of 87.6% of azophloxine was degraded in 30 min of irradiation. The photocatalytic reaction caused the degradation of the organic groups in the dye. The degradation efficiency in the first cycle was determined to be 87.7% in the solution using the Yb-K2Ti6O13(2000), and the degradation efficiency was 64.1% in the fifth cycle.
Graphical Abstract

Highlights
-
A sol-gel method was applied to prepare the porous ytterbium-doped potassium titanate.
-
PEG chain length could greatly influence the porous structure and particle size of the Yb-K2Ti6O13(n) samples.
-
The pores in the Yb-K2Ti6O13(n) samples became larger with a rising PEG chain length.
-
The largest BET surface area and pore volume were obtained for the Yb-K2Ti6O13(2000).
-
The largest number of hydroxyl radicals were generated by the Yb-K2Ti6O13(2000).







Similar content being viewed by others
References
Barceló D, Žonja B, Ginebreda A (2020) Toxicity tests in wastewater and drinking water treatment processes: A complementary assessment tool to be on your radar. J Environ Chem Eng 8:104262
Lu HB, Yu Y, Zhou YX, Xing F (2019) A quantitative evaluation method for wastewater toxicity based on a microbial fuel cell. Ecotoxicol Environ Saf 183:109589
Zhao WT, Sui Q, Huang X (2018) Removal and fate of polycyclic aromatic hydrocarbons in a hybrid anaerobic–anoxic–oxic process for highly toxic coke wastewater treatment. Sci Total Environ 635:716–724
Zan J, Song H, Zuo SY, Chen XR, Xia DS, Li DY (2020) MIL-53(Fe)-derived Fe2O3 with oxygen vacancy as Fenton-like photocatalysts for the elimination of toxic organics in wastewater. J Clean Prod 246:118971
Meftahi M, Jafari SH, Habibi-Rezaei M (2023) Fabrication of Mo-doped TiO2 nanotube arrays photocatalysts: The effect of Mo dopant addition time to an aqueous electrolyte on the structure and photocatalytic activity. Ceram Int 49:11411–11422
Dong SY, Cui LF, Zhang W, Xia LJ, Zhou SJ, Russell CK, Fan MH, Feng JL, Sun JH (2020) Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem Eng J 384:123279
Zhang KN, Zhang YS, Zhang DM, Liu CH, Zhou XX, Yang H, Qu J, He DY (2023) Efficient photocatalytic water disinfection by a novel BP/BiOBr S-scheme heterojunction photocatalyst. Chem Eng J 468:143581
Ambigadevi J, Senthil Kumar P, Dai-Viet NV, Hari Haran S, Srinivasa Raghavan TN (2021) Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A Review. J Environ Chem Eng 9:104881
Rasheed T, Adeel M, Nabeel F, Bilal M, Iqbal HMN (2019) TiO2/SiO2 decorated carbon nanostructured materials as a multifunctional platform for emerging pollutants removal. Sci Total Environ 688:299–311
Hendrix Y, Lazaro A, Yu QL, Brouwers HJH (2019) Influence of synthesis conditions on the properties of photocatalytic titania-silica composites. J Photochem Photobio A Chem 371:25–32
Zhu XD, Zhou Q, Xia YW, Wang J, Chen HJ, Xu Q, Liu JW, Feng W, Chen SH (2021) Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J Mater Sci Mater Electron 32:21511–21524
Zhu XD, Wang J, Yang DX, Liu JW, He LL, Tang M, Feng W, Wu XQ (2021) Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv 11:27257–27266
Barrocas B, Chiavassa LD, Oliveira MC, Monteiro OC (2020) Impact of Fe, Mn co-doping in titanate nanowires photocatalytic performance for emergent organic pollutants removal. Chemosphere 250:126240
Lu JX, Li DL, Chai Y, Li L, Li M, Zhang YY, Liang J (2019) Rational design and preparation of nanoheterostructures based on zinc titanate for solar-driven photocatalytic conversion of CO2 to valuable fuels. Appl Catal B Environ 256:117800
Rahut S, Panda R, Basu JK (2017) Solvothermal synthesis of a layered titanate nanosheets and its photocatalytic activity: Effect of Ag doping. J Photochem Photobio A Chem 341:12–19
Liu GG, Han K, Ye HQ, Zhu CY, Gao YP, Liu Y, Zhou YH (2017) Graphene oxide/triethanolamine modified titanate nanowires as photocatalytic membrane for water treatment. Chem Eng J 320:74–80
Tsai CC, Chen LC, Yeh TF, Teng H (2013) In situ Sn2+-incorporation synthesis of titanate nanotubes for photocatalytic dye degradation under visible light illumination. J Alloy Compd 546:95–101
Ibarra-Rodriguez LI, Garay-Rodríguez LF, Torres-Martínez LM (2021) Photocatalytic reduction of CO2 over K2Ti6O13 films. Mater Chem Phys 270:124836
Garay-Rodríguez LF, Torres-Martínez LM, Moctezuma E (2019) Photocatalytic performance of K2Ti6O13 whiskers to H2 evolution and CO2 photo-reduction. J Energy Chem 37:18–28
Zhu MY, Cai YW, Liu SY, Fang M, Tan XL, Liu XY, Kong MG, Xu W, Mei HY, Hayat T (2019) K2Ti6O13 hybridized graphene oxide: Effective enhancement in photodegradation of RhB and photoreduction of U(VI). Environ Pollut 248:448–455
Amornpitoksuk P, Suwanboon S, Kaowphong S, Randorn C, Graidist P (2020) Photocatalytic activity of K2Ti6O13/TiO2 nanocomposite prepared using water extract of wood ash from waste for degradation of dye pollutants. J Taiwan Inst Chem Eng 117:242–251
Balu K, Chicardi E, Sepúlveda R, Durai M, Ishaque F, Chauhan D, Ahn YH (2023) BiOX (X = I or Cl?) modified Na-K2Ti6O13 nanostructured materials for efficient degradation of Tetracycline, Acid Black 1 dye and microbial disinfection in wastewater under Blue LED. Sep Purif Technol 309:122998
Morozov NA, Ershov DS, Sinel’shchikova OY, Besprozvannykh NV, Mjakin SV, E.Yu. Brazovskaya OL, Galankina AV, Koroleva (2024) Effect of magnesium additive on acid-base properties and photocatalytic activity of nanostructured potassium titanate. Appl Surf Sci 644:158765
Zhang WJ, Ma Z, Du L, Li H (2017) Role of PEG4000 in sol-gel synthesis of Sm2Ti2O7 photocatalyst for enhanced activity. J Alloy Compds 704:26–31
Onozuka K, Kawakami Y, Imai H, Yokoi T, Tatsumi T, Kondo JN (2012) Perovskite-type La2Ti2O7 mesoporous photocatalyst. J Solid State Chem 192:87–92
Zhu XQ, Zhang JL, Chen F (2010) Hydrothermal synthesis of nanostructures Bi12TiO20 and their photocatalytic activity on acid orange 7 under visible light. Chemosphere 78:1350–1355
Hoffmann MR, Martin ST, Choi W, Bahnemann W (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Acknowledgements
This work was supported by Applied Basic Research Program of Liaoning Province (2023JH2/101300021), and Scientific Research Fund of Liaoning Provincial Education Department (LJKMZ20220594).
Author information
Authors and Affiliations
Contributions
Yi Zhao and Wenjie Zhang wrote the main manuscript text and Zhao Lv prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Zhang, W. & Lv, Z. Sol-gel synthesized porous Yb-K2Ti6O13(n) for photocatalytic degradation of azophloxine: effect of polyethylene glycol chain length. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06412-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06412-x