Abstract
Terbium orthoferrite (TbFeO3) perovskite demonstrates specific magnetic properties which are object of interest. Aggregated granular nanopowder of TbFeO3 particles of 85.6 nm were obtained by direct solution combustion method. The orthorhombic perovskite structure (space group Pbnm) of the terbium orthoferrite was confirmed by powder X-ray diffraction, Raman spectroscopy and 57Fe Mössbauer spectroscopy. The unusual granular morphology and mesoporous structure of the nanopowder were investigated by scanning electron microscopy and adsorption-structural analysis via low-temperature (77 K) adsorption-desorption of nitrogen with a total porosity of 0.0145 cm³/g and average pore width of 12 nm. The magnetic properties of TbFeO3 nanoparticles were thoughtfully studied, M = 34.2 emu/g at T = 10 K, Hc = 300 Oe. The onset of reorientation of the Fe3+ spin system at T ~ 18 K and the antiferromagnetic ordering of Tb3+ ions at T ~ 4 K were observed by vibrational magnetometry. Obtained results confirm the flexible paramagnetic to antiferromagnetic behavior of granular TbFeO3 nanopowder.
Graphical Abstract

Highlight
-
Granular nanopowder of TbFeO3 was obtain by direct solution combustion method.
-
Aggregated granular morphology of perovskite TbFeO3 was confirmed.
-
Magnetization of TbFeO3 granules is in pronounced dependence of temperature.






Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Žužić A, Ressler A, Macan J (2022) Perovskite oxides as active materials in novel alternatives to well-known technologies: A review. Ceram Int 48:27240–27261. https://doi.org/10.1016/J.CERAMINT.2022.06.152
Stanislavchuk TN, Wang Y, Cheong SW, Sirenko AA (2017) Far-IR magnetospectroscopy of magnons and electromagnons in TbFeO3 single crystals at low temperatures. Phys Rev B 95:1–11. https://doi.org/10.1103/PhysRevB.95.054427
Zou YH, Li WL, Wang SL et al. (2012) Spin dependent electrical abnormal in TbFeO3. J Alloy Compd 519:82–84. https://doi.org/10.1016/j.jallcom.2011.12.058
Martinson KD, Ivanov AA, Panteleev IB, Popkov VI (2021) Effect of sintering temperature on the synthesis of LiZnMnFe microwave ceramics with controllable electro/magnetic properties. Ceram Int 47:30071–30081. https://doi.org/10.1016/j.ceramint.2021.07.183
Martinson KD, Beliaeva AD, Sakhno DD et al. (2022) Synthesis, Structure, and Antimicrobial Performance of Nix Zn1−x Fe2 O4 (x = 0, 0.3, 0.7, 1.0) Magnetic Powders toward E. coli, B. cereus, S. citreus, and C. tropicalis. Water (Switz) 14:1–17. https://doi.org/10.3390/w14030454
Baladi M, Amiri M, Akbari Javar H et al. (2022) Green synthesis of perovskite-type TbFeO3/CuO as a highly efficient modifier for electrochemical detection of methyldopa. J Electroanal Chem 915:116339. https://doi.org/10.1016/J.JELECHEM.2022.116339
Shifrina ZB, Bronstein LM (2018) Magnetically Recoverable Catalysts. Beyond Magn Sep 6:1–6. https://doi.org/10.3389/fchem.2018.00298
Zhao S, Luo Y, Li C et al. (2023) High-performance photothermal catalytic CO2 reduction to CH4 and CO by ABO3 (A = La, Ce; B = Ni, Co, Fe) perovskite nanomaterials. Ceram Int 49:20907–20919. https://doi.org/10.1016/J.CERAMINT.2023.03.224
Bidrawn F, Kim G, Aramrueang N et al. (2010) Dopants to enhance SOFC cathodes based on Sr-doped LaFeO3 and LaMnO3. J Power Sources 195:720–728. https://doi.org/10.1016/j.jpowsour.2009.08.034
Li B, Irvine JTS, Ni J, Ni C (2022) High-performance and durable alcohol-fueled symmetrical solid oxide fuel cell based on ferrite perovskite electrode. Appl Energy 306:118117. https://doi.org/10.1016/J.APENERGY.2021.118117
Yoshii K, Mizumaki M, Matsumoto K, et al. (2013) Magnetic properties of single crystalline YbFe2O4. J Phys Conf Ser 428:. https://doi.org/10.1088/1742-6596/428/1/012032
Mehdizadeh P, Masjedi-Arani M, Amiri O, Salavati-Niasari M (2021) Rapid microwave fabrication of new nanocomposites based on Tb-Fe-O nanostructures for electrochemical hydrogen storage application. Fuel 304:121412. https://doi.org/10.1016/j.fuel.2021.121412
Sivakumar M, Gedanken A, Bhattacharya D et al. (2004) Sonochemical synthesis of nanocrystalline rare earth orthoferrites using Fe(CO)5 precursor. Chem Mater 16:3623–3632. https://doi.org/10.1021/cm049345x
Belakhovsky M, Chappert J, T Rouskov JS (1982) Successive reorientations of iron moments in YbFeO3, TbFeO3 and ErFeO3. J Phys Colloq 43:C5-5491–C5-5503. https://doi.org/10.1051/jphyscol:19711162
Park BG, Kim SB, Lee HJ et al. (2008) Magnetic properties of the orthoferrites TbFeO3 and ErFeO3. J Korean Phys Soc 53:758–762. https://doi.org/10.3938/jkps.53.758
Vilarinho R, Weber MC, Guennou M et al. (2022) Magnetostructural coupling in RFeO3 (R = Nd, Tb, Eu and Gd). Sci Rep 12:1–14. https://doi.org/10.1038/s41598-022-13097-1
Song YQ, Zhou WP, Fang Y, et al. (2014) Multiferroic properties in terbium orthoferrite. Chinese Phys B 23:. https://doi.org/10.1088/1674-1056/23/7/077505
Ovsianikov AK, Usmanov OV, Zobkalo IA et al. (2022) Inelastic neutron studies and diffraction in magnetic fields of TbFeO3 and YbFeO3. J Magn Magn Mater 563:170025. https://doi.org/10.1016/J.JMMM.2022.170025
Karaki MJ, Yang X, Williams AJ et al. (2023) An efficient material search for room temperature topological magnons. Sci Adv 9:eade773. https://doi.org/10.48550/arXiv.2206.06248
Popkov VI, Tugova EA, Bachina AK, Almyasheva OV (2017) The formation of nanocrystalline orthoferrites of rare-earth elements XFeO3 (X = Y, La, Gd) via heat treatment of coprecipitated hydroxides. Russ J Gen Chem 87:2516–2524. https://doi.org/10.1134/S1070363217110020
Gupta P, Mahapatra PK, Choudhary RNP (2020) TbFeO3 Ceramic: An Exciting Colossal Dielectric with Ferroelectric Properties. Phys Status Solidi Basic Res 257:1–14. https://doi.org/10.1002/pssb.201900236
Luo D, Wang P, Zheng Q et al. (2021) Magnetic nanoparticle-based solid phase peptide synthesis and the synchronous detection of their biological activity. Mater Today Adv 12:100175. https://doi.org/10.1016/j.mtadv.2021.100175
Konishi S, Oka K, Eisaki H et al. (2019) Growth of Single-Crystalline RFe2O4-Δ (R = Y, Tm, Yb) by the Floating Zone Melting Method in a Mixture of N2, H2, and CO2 Gases and Magnetic Properties of the Compounds. Cryst Growth Des 19:5498–5504. https://doi.org/10.1021/acs.cgd.8b01393
Berezhnaya MV, Mittova IY, Perov NS et al. (2018) Production of Zinc-Doped Yttrium Ferrite Nanopowders by the Sol–Gel Method. Russ J Inorg Chem 63:742–746. https://doi.org/10.1134/S0036023618060049
Tang PS, Wang ZH, Ying JN et al. (2015) One-step preparation of nanoparticulate TbFeO3 by microwave process and its visible-light photocatalytic activity. Mater Sci Forum 809–810:109–113
Yang H, Zhang JX, Lin GJ et al. (2013) Preparation, characterization and photocatalytic properties of terbium orthoferrite nanopowder. Adv Powder Technol 24:242–245. https://doi.org/10.1016/j.apt.2012.06.009
Martinson KD, Ivanov VA, Chebanenko MI et al. (2019) Facile combustion synthesis of TbFeO3 nanocrystals with hexagonal and orthorhombic structure. Nanosyst Phys, Chem Math 10:694–700. https://doi.org/10.17586/2220-8054-2019-10-6-694-700
Popkov VI, Martinson KD, Kondrashkova IS, et al. (2021) SCS-assisted production of EuFeO3 core-shell nanoparticles: formation process, structural features and magnetic behavior. J Alloys Compd 859:. https://doi.org/10.1016/j.jallcom.2020.157812
Gimaztdinova MM, Tugova EA, Tomkovich MVPVI (2016) Synthesis of GdFeO3 nanocrystals via glycine-nitrate combustion. Condens Phases Interfaces 18:422–431
Tikhanova SM, Lebedev LA, Martinson KD et al. (2021) The synthesis of novel heterojunction h-YbFeO3/o-YbFeO3 photocatalyst with enhanced Fenton-like activity under visible-light. N. J Chem 45:1541–1550. https://doi.org/10.1039/d0nj04895j
AS Seroglazova, MI Chebanenko VIP (2021) Synthesis, structure, and photo-Fenton activity of PrFeO3-TiO2 mesoporous nanocomposites. 23:3–15. https://doi.org/10.17308/kcmf.2021.23/3674
Martinson KD, Kondrashkova IS, Omarov SO et al. (2020) Magnetically recoverable catalyst based on porous nanocrystalline HoFeO3 for processes of n-hexane conversion. Adv Powder Technol 31:402–408. https://doi.org/10.1016/j.apt.2019.10.033
Popkov VI, Almjasheva OV, Nevedomskiy VN et al. (2018) Effect of spatial constraints on the phase evolution of YFeO3-based nanopowders under heat treatment of glycine-nitrate combustion products. Ceram Int 44:20906–20912. https://doi.org/10.1016/j.ceramint.2018.08.097
Zhou Z, Guo L, Yang H et al. (2014) Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J Alloy Compd 583:21–31. https://doi.org/10.1016/j.jallcom.2013.08.129
Fortuño-Morte M, Serna-Gallén P, Beltrán-Mir H, Cordoncillo E (2021) A new series of environment-friendly reddish inorganic pigments based on AFeO3 (A = Ln, Y) with high NIR solar reflectance. J Mater 7:1061–1073. https://doi.org/10.1016/j.jmat.2021.02.002
Iliev M, Abrashev M (1998) Raman spectroscopy of orthorhombic perovskitelike and. Phys Rev B - Condens Matter Mater Phys 57:2872–2877. https://doi.org/10.1103/PhysRevB.57.2872
Vilarinho R, Weber MC, Guennou M et al. (2022) Magnetostructural coupling in RFeO3 (R = Nd, Tb, Eu and Gd). Sci Rep. 12:1–15. https://doi.org/10.1038/s41598-022-13097-1
Lazarević Z, Jovalekić Č, Gilić M et al. (2017) Yttrium orthoferrite powder obtained by the mechanochemical synthesis. Sci Sinter 49:277–284. https://doi.org/10.2298/SOS1703277L
Brown SR, Hall I (1993) Mossbauer study of field-driven spin reorientations in YbFeO3 at 4.2 K. J Phys Condens Matter 5:4207–4214. https://doi.org/10.1088/0953-8984/5/25/010
Alsowayigh MM, Timco GA, Borilovic I et al. (2020) Heterometallic 3d-4f complexes as air-stable molecular precursors in low temperature syntheses of stoichiometric rare-earth orthoferrite powders. Inorg Chem 59:15796–15806. https://doi.org/10.1021/acs.inorgchem.0c02249
Pinho SLC, Amaral JS, Wattiaux A et al. (2018) Synthesis and Characterization of Rare-Earth Orthoferrite LnFeO3 Nanoparticles for Bioimaging. Eur J Inorg Chem 2018:3570–3578. https://doi.org/10.1002/ejic.201800468
Tejada J, Zhang XX, Roig A et al. (1995) Quantum tunnelling of antiferromagnetic domain walls in TbFeO3 single crystal. Epl 30:227–232. https://doi.org/10.1209/0295-5075/30/4/007
Tikhanova SM, Seroglazova AS, Buryanenko IV, et al. (2023) Synthesis and phase transformations of hexagonal, orthorhombic, and cubic ScxLu1-xFeO3 (0 ≤ x ≤ 1) nanocrystals. Ceram Int. https://doi.org/10.1016/j.ceramint.2023.09.350
Acknowledgements
Authors are greatly appreciating to Andrey Trofimuk for Raman measurements carried out on Integra spectra system in Laboratory for Cluster Structures of Ioffe institute.
Author information
Authors and Affiliations
Contributions
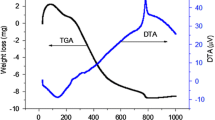
SMT - wrote original draft, ASS - analysed data and prepared figure 1, MIC - analysed and interpretated data for figure 4, VVP, VGS - prepared figure 3, MVP - analysed data and prepared figures 5-6, wrote original draft, VNN - analysed and interpretated data for figure 2, VIP - supervised and reviewed the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tikhanova, S.M., Seroglazova, A.S., Chebanenko, M.I. et al. Structural, morphological, and magnetic features of granular TbFeO3 perovskite synthesized via direct solution combustion synthesis. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06407-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06407-8