Abstract
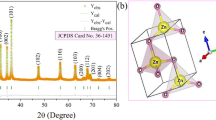
Three zinc oxide catalysts with different morphologies are synthesized by the sol-gel method. Zinc acetate and hexamethylenetetramine (HMTA) are used to produce nanorods (NR) and nanodiscs (ND). ZnO nanoflowers (NF) are produced from different reactants, namely zinc nitrate and sodium hydroxide. The photocatalysts are efficient for degrading p-nitrophenol under halogen lamp illumination (300–800 nm). X-ray diffraction confirms the presence of the wurtzite structure, and scanning electron microscopy confirms the desired morphologies. In order to understand the differences in the kinetic rate of degradation between the three catalysts, surface defects are investigated using photoluminescence and Raman spectroscopy. Moreover, colloidal stability and specific surface area are determined by zeta potential and nitrogen sorption measurements, respectively, and allow the impact of the different parameters on the photocatalytic performance of the samples to be clearly understood. Although they do not have the highest number of defects nor the largest specific surface area, ND shows the best degradation results by reaching 75% of degradation after 8 h. This result can be attributed to the morphology of this catalyst, where the polar facets are exposed to the medium and play a crucial role in the photocatalytic performance by enhancing the lifetime of the electron/hole pairs generated upon illumination. The polar nature of both catalyst and pollutant increases the contact between them and, consequently, the degradation efficiency.
Graphical Abstract

Controlling the morphology of ZnO allows better degradation of p-nitrophenol under UV-vis. Three morphologies were compared, and the nanodiscs showed the best photocatalytic activity.
Highlights
-
Nanorods, nanodiscs and nanoflowers zinc oxides are synthesized by sol-gel method.
-
X-ray diffraction confirms the presence of the photoactive wurtzite structure.
-
The photocatalysts are efficient to degrade p-nitrophenol under UV/visible illumination.
-
The best degradation result is obtained with the nanodiscs (ND) by reaching 75% of degradation after 8 h.
-
ND show high polar facets exposition leading to a longer lifetime of photogenerated species.










Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as these data are part of an ongoing study.
References
Kumar Reddy DH, Lee SM (2012) Water Pollution and Treatment Technologies. J Environ Anal Toxicol 02: https://doi.org/10.4172/2161-0525.1000e103
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155
Dewil R, Mantzavinos D, Poulios I, Rodrigo MA (2017) New perspectives for advanced oxidation processes. J Environ Manag 195:93–99
Mahy JG, Wolfs C, Mertes A et al. (2019) Advanced photocatalytic oxidation processes for micropollutant elimination from municipal and industrial water. J Environ Manage 250: https://doi.org/10.1016/j.jenvman.2019.109561
Mahy JG, Paez CA, Carcel C et al. (2019) Porphyrin-based hybrid silica-titania as a visible-light photocatalyst. J Photochem Photobio A Chem 373:66–76. https://doi.org/10.1016/j.jphotochem.2019.01.001
Nawrocki J, Kasprzyk-Hordern B (2010) The efficiency and mechanisms of catalytic ozonation. Appl Catal B 99:27–42
Babuponnusami A, Muthukumar K (2014) A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng 2:557–572
Lama G, Meijide J, Sanromán A, Pazos M (2022) Heterogeneous advanced oxidation processes: current approaches for wastewater treatment. Catalysts 12:344
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res 88:428–448
Flores NM, Pal U, Galeazzi R, Sandoval A (2014) Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv 4:41099–41110. https://doi.org/10.1039/c4ra04522j
Yang J, Wang J, Li X et al. (2012) Effect of polar and non-polar surfaces of ZnO nanostructures on photocatalytic properties. J Alloy Compd 528:28–33. https://doi.org/10.1016/j.jallcom.2012.02.162
Gan YX, Jayatissa AH, Yu Z et al. (2020) Hydrothermal synthesis of nanomaterials. J Nanomater 2020:8917013
Lai J, Niu W, Luque R, Xu G (2015) Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 10:240–267
Kumar A, Kuang Y, Liang Z, Sun X (2020) Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater Today Nano 11:100076
Sajjadi SP (2005) Sol-gel process and its application in Nanotechnology. J Polym Eng Technol 13:38–41
Lambert S, Tran KY, Arrachart G et al. (2008) Tailor-made morphologies for Pd/SiO2 catalysts through sol-gel process with various silylated ligands. Microporous Mesoporous Mater 115:609–617. https://doi.org/10.1016/j.micromeso.2008.03.003
Brinker CJ, Scherer GW. Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, 2013
Hu J, Ding J, Ai J et al. (2021) Room temperature growth of ZnO with highly active exposed facets for photocatalytic application. Nanotechnol Rev 10:919–932. https://doi.org/10.1515/ntrev-2021-0057
Mahy JG, Deschamps F, Collard V et al. (2018) Acid acting as redispersing agent to form stable colloids from photoactive crystalline aqueous sol–gel TiO2 powder. J Solgel Sci Technol 87:568–583. https://doi.org/10.1007/s10971-018-4751-6
Luo S, Chen R, Xiang L, Wang J (2019) Hydrothermal synthesis of (001) facet highly exposed ZnO plates: a new insight into the effect of citrate. Crystals 9:. https://doi.org/10.3390/cryst9110552
Wöll C (2007) The chemistry and physics of zinc oxide surfaces. Prog Surf Sci 82:55–120
Abubakar S, Tan ST, Liew JYC et al. (2023) Controlled growth of semiconducting ZnO nanorods for piezoelectric energy harvesting-based nanogenerators. Nanomaterials 13:1025. https://doi.org/10.3390/nano13061025
Wang M, Zhou Y, Zhang Y et al. (2011) From Zn(OH)2 to ZnO: a study on the mechanism of phase transformation. CrystEngComm 13:6024–6026. https://doi.org/10.1039/c1ce05502j
McCluskey MD, Jokela SJ (2009) Defects in ZnO. J Appl Phys 106. https://doi.org/10.1063/1.3216464
Wang M, Zhou Y, Zhang Y et al. (2012) Near-infrared photoluminescence from ZnO. Appl Phys Lett 100. https://doi.org/10.1063/1.3692584
Janotti A, Van De Walle CG (2009) Fundamentals of zinc oxide as a semiconductor. Rep Prog Phys 72. https://doi.org/10.1088/0034-4885/72/12/126501
Huang Y, Yu Y, Yu Y, Zhang B (2020) Oxygen vacancy engineering in photocatalysis. Solar RRL 4:2000037
Russo V, Ghidelli M, Gondoni P et al. (2014) Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J Appl Phys 115. https://doi.org/10.1063/1.4866322
Song Y, Zhang S, Zhang C et al. (2019) Raman spectra and microstructure of zinc oxide irradiated with swift heavy ion. Crystals 9:. https://doi.org/10.3390/cryst9080395
Herrmann JM (2010) Fundamentals and misconceptions in photocatalysis. J Photochem Photobio A Chem 216:85–93
Azam A, Babkair SS (2014) Low-temperature growth of well-aligned zinc oxide nanorod arrays on silicon substrate and their photocatalytic application. Int J Nanomed 9:2109–2115. https://doi.org/10.2147/IJN.S60839
Vargas R, Núñez O (2009) Hydrogen bond interactions at the TiO2 surface: their contribution to the pH dependent photo-catalytic degradation of p-nitrophenol. J Mol Catal A Chem 300:65–71. https://doi.org/10.1016/j.molcata.2008.10.029
Tchieno FMM, Tonle IK (2018) P-Nitrophenol determination and remediation: an overview. Rev Anal Chem 37. https://doi.org/10.1515/revac-2017-0019
Chen Y, Zhao H, Liu B, Yang H (2015) Charge separation between wurtzite ZnO polar {001} surfaces and their enhanced photocatalytic activity. Appl Catal B 163:189–197. https://doi.org/10.1016/j.apcatb.2014.07.044
Huang M, Lian J, Si R et al. (2022) Spatial separation of electrons and holes among ZnO Polar {0001} and {101¯ 0} facets for enhanced photocatalytic performance. ACS Omega 7:26844–26852. https://doi.org/10.1021/acsomega.2c03244
Kayaci F, Vempati S, Donmez I et al. (2014) Role of zinc interstitials and oxygen vacancies of ZnO in photocatalysis: a bottom-up approach to control defect density. Nanoscale 6:10224–10234. https://doi.org/10.1039/c4nr01887g
Acknowledgements
JGM, SH and SDL thank the F.R.S.-FNRS for his Postdoctoral Researcher position and their Research Director position, respectively. JGM is also grateful to the Rotary for a District 2160 grant, to the University of Liège and the FNRS for financial support for a postdoctoral stay in INRS Centre Eau, Terre, Environnement in Québec, Canada. The authors would like to thank the CARPOR platform of the University of Liège as well as its manager Dr. A. Léonard for the assistance in gas adsorption measurements.
Funding
For their financial support, the authors are grateful to the Ministère de la Région Wallonne Direction Générale des Technologies, de la Recherche et de l’Energie (DGO6) with support from the “31st CORNET Call” funds for the research project “DAF3D - Development of new antibacterial functionalized textiles and 3-D-printed filters for process water treatment”.
Author information
Authors and Affiliations
Contributions
AF: Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft Writing—review & editing. SDL: Conceptualization, Methodology, Writing—review & editing, Funding acquisition and project administration. DP: Investigation, Formal analysis, Writing—review & editing. ZY: Investigation, Formal analysis, Writing—review & editing Fabien Drault: Investigation, Formal analysis, Writing—review & editing. SH: Investigation, Formal analysis, Writing—review & editing. PD: Investigation, Formal analysis, Writing—review & editing. BH: Methodology, Writing—review & editing, Funding acquisition. CM: Investigation, Formal analysis, Writing—review & editing. GE: Investigation, Formal analysis, Writing—review & editing. AV: Investigation, Formal analysis, Writing—review & editing. JGM: Investigation, Formal analysis, Methodology, Writing—review & editing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
All authors agreed to participate in this work.
Consent to publish
All authors agreed to this version for publication.
Ethical approval
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farcy, A., Lambert, S.D., Poelman, D. et al. Influence of crystallographic facet orientations of sol-gel ZnO on the photocatalytic degradation of p-nitrophenol in water. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-023-06301-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-023-06301-9