Abstract
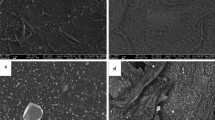
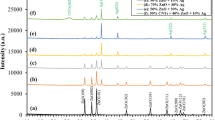
One novel method for disinfecting water is the use of silver nanoparticles. The aim of this work was to prepare a granular antibacterial composite based on chitosan boehmite, enhanced with silver nanoparticles (Ag-CHB), and evaluate its antibacterial activity. The composite was prepared by the sol-gel method and characterized by FTIR, AFM, FESEM, EDS, and UV-VIS techniques. The topographical study using the AFM revealed a clear change in the surface shape of the composite after it was reinforced with silver nanoparticles. Additionally, FESEM images displayed that the particles of chitosan-boehmite supported with silver nanoparticles are polygonal granules with an average size of 61.93 nm. The EDX analysis showed that the percentage of silver atoms in the composite is about 1.24 wt%. The results of FTIR indicated that chitosan’s functional groups have specific interactions with silver and aluminum. According to XRD analysis, boehmite nanocrystals are orthorhombic, with a mean crystal size of 30 nm was confirmed. Optical characterization of the colloidal silver nanoparticles by UV-VIS technique revealed the presence of a clear surface plasmon band, with a peak wavelength of 389 nm. The stability in the water of the prepared composite was verified by the swelling test, and the swelling index was about 11.82%. The ability of the composite to kill bacteria was also verified before and after being reinforced with silver nanoparticles by the agar well diffusion method. The composite reinforced with silver nanoparticles showed antibacterial activity against E. coli, with a 10 mm inhibition zone diameter. Overall, these results indicated that the Ag-CHB composite has the potential for water disinfection and incorporation into household filtration systems.
Graphical Abstract

Highlights
-
Chitosan-boehmite, enhanced with silver nanoparticles, is synthesized by the sol-gel method.
-
The successful formation of the composite was corroborated through several techniques.
-
The composite exhibits full stability in water and demonstrates antibacterial properties.
-
Chitosan-boehmite enhanced with silver nanoparticles granules, can be used in multimedia household filters.











Similar content being viewed by others
Abbreviations
- CHB:
-
chitosan boehmite
- Ag-CHB:
-
chitosan boehmite enhanced with silver nanoparticles
- BRE%:
-
bacterial removal efficiency
- SI%:
-
swelling index
References
World Health Organization (WHO)(eds) (2005) The evidence to support hygiene, sanitation and water in schools. In: Water quality: guidelines, standards and health, vol. 23, no. 3. IWA, London, UK, p 2–5. https://www.jstor.org/stable/24684611
Odonkor ST, Mahami T (2020) Escherichia coli as a tool for disease risk assessment of drinking water sources. Int J Microbiol. https://doi.org/10.1155/2020/2534130
Hunter PR (2003) Drinking water and diarrhoeal disease due to Escherichia coli. J Water Health. 1:65–72. https://doi.org/10.2166/wh.2003.0008
Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed 8:4467–4479. https://doi.org/10.2147/IJN.S50837
Sibuyi NRS et al. (2021) Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: a review. Nanoscale Res Lett 16:1. https://doi.org/10.1186/s11671-021-03632-w
Abebe B, Zereffa EA, Tadesse A, Murthy HCA (2020) A review on enhancing the antibacterial activity of ZnO: mechanisms and microscopic investigation. Nanoscale Res Lett 15:1. https://doi.org/10.1186/s11671-020-03418-6
Zein R, Alghoraibi I, Soukkarieh C, Ismail MT, Alahmad A (2022) Influence of polyvinylpyrrolidone concentration on properties and anti-bacterial activity of green synthesized silver nanoparticles. Micromachines 13:777. https://doi.org/10.3390/mi13050777
Franci G et al. (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20(5):8856–8874. https://doi.org/10.3390/molecules20058856
Lu L et al. (2008) Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther 13(2):252–262
Shepard ZJ, Lux EM, Oyanedel-Craver VA (2020) Performance of silver nanoparticle-impregnated ovoid ceramic water filters. Environ Sci Nano 7(6):1772–1780. https://doi.org/10.1039/d0en00115e
Ghodake G, Shinde S, Saratale GD, Kadam A, Saratale RG, Kim DY (2020) Water purification filter prepared by layer-by-layer assembly of paper filter and polypropylene-polyethylene woven fabrics decorated with silver nanoparticles. Fibers Polym 21(4):751–761. https://doi.org/10.1007/s12221-020-9624-2
Mollahosseini A, Rahimpour A, Jahamshahi M, Peyravi M, Khavarpour M (2012) The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 306:41–50. https://doi.org/10.1016/j.desal.2012.08.035
Yuan J, Geng J, Xing Z, Shen J, Kang I-K, Byun H (2009) Electrospinning of antibacterial poly(vinylidene fluoride) nanofibers containing silver nanoparticles. J Appl Polym Sci 116(5):669–671. https://doi.org/10.1002/app.31632
Mpenyana-Monyatsi L, Mthombeni NH, Onyango MS, Momba MNB (2012) Cost-effective filter materials coated with silver nanoparticles for the removal of pathogenic bacteria in groundwater. Int J Environ Res Public Health 9(1):244–271. https://doi.org/10.3390/ijerph9010244
Yao KM, Habibian MT, O’Melia CR (1971) Water and waste water filtration: concepts and applications. Environ Sci Technol 5(11):1105–1112. https://doi.org/10.1021/es60058a005
El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44(4):1260–1266. https://doi.org/10.1021/es902240k
Zhang H, Smith JA, Oyanedel-Craver V (2012) The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res 46(3):691–699. https://doi.org/10.1016/j.watres.2011.11.037
Lehtonen J et al. (2019) Effects of chloride concentration on the water disinfection performance of silver containing nanocellulose-based composites. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-56009-6
Yuan B, Sui M, Lu H, Wang J, Qin J (2019) The combined effect of light irradiation and chloride on the physicochemical properties of silver nanoparticles. RSC Adv 10(1):228–235. https://doi.org/10.1039/c9ra09261g
U.S. Environmental Protection Agency (2020) Drinking water regulations and contaminants. Safe Drinking Water Act (SDWA). https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants
Kumar S, Ye F, Dobretsov S, Dutta J (2019) Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl Sci 9:12. https://doi.org/10.3390/app9122409
Spoială A, Ilie CI, Ficai D, Ficai A, Andronescu E (2021) Chitosan-based nanocomposite polymeric membranes for water purification—a review. Materials 14(9):1–29. https://doi.org/10.3390/ma14092091
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004
Upadhyay U, Sreedhar I, Singh SA, Patel CM, Anitha KL (2021) Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr Polym 251:1–29. https://doi.org/10.1016/j.carbpol.2020.117000
Vatanpour V, Madaeni SS, Rajabi L, Zinadini S, Derakhshan AA (2012) Boehmite nanoparticles as a new nanofiller for preparation of antifouling mixed matrix membranes. J Membr Sci 401–402:132–143. https://doi.org/10.1016/j.memsci.2012.01.040
Ghasem Zadeh Khorasani M, Silbernagl D, Szymoniak P, Hodoroaba V-D, Sturm H (2019) The effect of boehmite nanoparticles (γ‐AlOOH) on nanomechanical and thermomechanical properties correlated to crosslinking density of epoxy. Polymer 164:174–182. https://doi.org/10.1016/j.polymer.2018.12.054
Dubey SP, Dwivedi AD, Sillanpää M, Lee H, Kwon YN, Lee C (2017) Adsorption of As(V) by boehmite and alumina of different morphologies prepared under hydrothermal conditions. Chemosphere 169:99–106. https://doi.org/10.1016/j.chemosphere.2016.11.052
Liu X, Niu C, Zhen X, Wang J, Su X (2015) Novel approach for synthesis of boehmite nanostructures and their conversion to aluminum oxide nanostructures for remove Congo red. J Colloid Interface Sci 452:116–125. https://doi.org/10.1016/j.jcis.2015.04.037
Jiménez-Becerril J, Solache-Ríos M, García-Sosa I (2012) Fluoride removal from aqueous solutions by boehmite. Water Air Soil Pollut 223(3):1073–1078. https://doi.org/10.1007/s11270-011-0925-3
Auxilio AR et al. (2008) Functionalised pseudo-boehmite nanoparticles as an excellent adsorbent material for anionic dyes. J Mater Chem 18(21):2466–2474. https://doi.org/10.1039/b715545j
Miao YE, Wang R, Chen D, Liu Z, Liu T (2012) Electrospun self-standing membrane of hierarchical SiO 2atγ -AlOOH (Boehmite) core/sheath fibers for water remediation. ACS Appl Mater Interfaces 4(10):5353–5359. https://doi.org/10.1021/am3012998
Saha G, Maliyekkal SM, Sabumon PC, Pradeep T (2015) A low cost approach to synthesize sand like AlOOH nanoarchitecture (SANA) and its application in defluoridation of water. J Environ Chem Eng 3(2):1303–1311. https://doi.org/10.1016/j.jece.2014.11.030
Rajamani M, Rajendrakumar K (2019) Chitosan-boehmite desiccant composite as a promising adsorbent towards heavy metal removal. J Environ Manag 244:257–264. https://doi.org/10.1016/j.jenvman.2019.05.056
Chen Y, Cai W, Dang C, Fan J, Zhou J, Liu Z (2020) A facile sol–gel synthesis of chitosan–boehmite film with excellent acid resistance and adsorption performance for Pb(II). Chem Eng Res Des 161:332–339. https://doi.org/10.1016/j.cherd.2020.07.018
Sankar MU et al. (2013) Biopolymer-reinforced synthetic granular nanocomposites for affordable point-of-use water purification. Proc Natl Acad Sci 110:8459–8464. https://doi.org/10.1073/pnas.1220222110
Iwai S-I, Yamamoto H, Morikawa H, Isobe M (1973) Topotactic thermal-transformation of diaspore to corundum. Mineral J 7(2):137–158. https://doi.org/10.2465/minerj1953.7.137
Thamilarasan V et al. (2018) Single step fabrication of chitosan nanocrystals using penaeus semisulcatus: potential as new insecticides, antimicrobials and plant growth promoters. J Clust Sci 29:375–384. https://doi.org/10.1007/s10876-018-1342-1
Paramelle D, Sadovoy A, Gorelik S, Free P, Hobley J, Fernig DG (2014) A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 139(19):4855–4861. https://doi.org/10.1039/c4an00978a
Zúñiga-Zamora A, García-Mena J, Cervantes-González E (2016) Removal of Congo Red from the aqueous phase by chitin and chitosan from waste shrimp. Desalin Water Treat 57:14674–14685. https://doi.org/10.1080/19443994.2015.1065444
Jafar Tafreshi M, Masoomi Khanghah Z (2015) Infrared spectroscopy studies on sol-gel prepared alumina powders. Mater Sci 21. https://doi.org/10.5755/j01.ms.21.1.4872
Sun T, Zhuo Q, Chen Y, Wu Z (2015) Synthesis of boehmite and its effect on flame retardancy of epoxy resin. High Perform Polym 27:100–104. https://doi.org/10.1177/0954008314540312
Fornaro T, Burini D, Biczysko M, Barone V (2015) Hydrogen-bonding effects on infrared spectra from anharmonic computations: uracil–water complexes and uracil dimers. J Phys Chem A 119:4224–4236. https://doi.org/10.1021/acs.jpca.5b01561
Kumar-Krishnan S et al. (2015) Chitosan/silver nanocomposites: synergistic antibacterial action of silver nanoparticles and silver ions. Eur Polym J 67:242–251. https://doi.org/10.1016/j.eurpolymj.2015.03.066
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Mikhailova EO (2020) Silver nanoparticles: mechanism of action and probable bio-application. J Funct Biomater 11:84. https://doi.org/10.3390/jfb11040084
Ho CM, Wong CK, Yau SKW, Lok CN, Che CM (2011) Oxidative dissolution of silver nanoparticles by dioxygen: a kinetic and mechanistic study. Chem Asian J 6(9):2506–2511. https://doi.org/10.1002/asia.201100034
Molleman B, Hiemstra T (2015) Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir 31:13361–13372. https://doi.org/10.1021/acs.langmuir.5b03686
Chen X, Yu L, Zou S, Xiao L, Fan J (2020) Zeolite cotton in tube: a simple robust household water treatment filter for heavy metal removal. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-61776-8
Nangmenyi G, Xao W, Mehrabi S, Mintz E, Economy J (2009) Bactericidal activity of Ag nanoparticle-impregnated fibreglass for water disinfection. J Water Health 7:657–663. https://doi.org/10.2166/wh.2009.107
Author information
Authors and Affiliations
Contributions
Both authors prepared and characterized the materials. FH wrote the manuscript, and designed the figures. Both authors revised and improved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasan, F., Alghoraibi, I. Chitosan-boehmite reinforced AgNPs prepared by the sol-gel method for cost-effective water disinfection. J Sol-Gel Sci Technol 109, 226–236 (2024). https://doi.org/10.1007/s10971-023-06266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06266-9