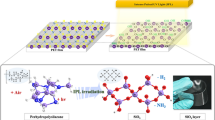
Abstract
In the previous study, prebaked films are prepared from positive-type photosensitive oligomeric silsesquioxanes with phenyl (>50 mol%) and methyl substituents containing a diazonaphthoquinone derivative. Subsequently, forward-tapered patterns with <10-µm resolution widths are created from the films through mask exposure and alkali development. However, transparent insulating films used in optical devices require chemical and heat resistance together with gently forward-tapered shapes to prevent disconnection of the upper-layer wirings. In the present work, both prebaked and developed films are subjected to bleaching exposure and subsequent post-baking to clarify the effects of these treatments on the film properties. The treatments are found to complete the condensation reactions to form films with high visible-range transparency and good chemical and heat (230 °C) resistance. Notably, flow, fusion, and wrinkling occur during post-baking, resulting in desirable gentle-shaped patterns. The minimum pattern widths decrease from 40 to 20 µm with increasing methyl group content. Infrared absorption spectroscopy and thermogravimetric analyses reveal that the decrease in width is due to the increased fraction of regular ladder structures generated by condensation reactions during post-baking. The increase in highly reactive methyl-substituted silanol groups results in lower molecular chain motion at lower temperatures, leading to an increase in this fraction.
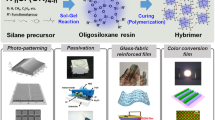
Graphical Abstract

a Structural formulae of oligo- and poly-SQs (A)–(C). b Process for transparent cured film pattern from positive-type photosensitive coatings. c Patterning performance of photosensitive oligo-SQs. d IR absorption spectra of bleach-cured films. e TG curves of bleached films
Highlights
-
Cured films and patterns were formed via a transparent curing treatment of developed films and patterns obtained from sol-gel-derived positive-type photosensitive oligomeric silsesquioxanes.
-
The films exhibited high visible-range transparency and good chemical and heat (230 °C) resistance, and all patterns had gently forward-tapered shapes.
-
The minimum pattern widths decreased from 40 to 20 μm with increasing methyl group content in the oligomeric silsesquioxanes.
-
The decrease in width was attributed to the lower molecular chain motion, which resulted from the increase in the fraction of regular ladder structures generated at low temperatures by condensation reactions during post-baking.










Similar content being viewed by others
References
Suzuki M, Sasaki H, Nishimura I, Yonezawa F, Endo M, Niwa K (2004) Radiation-sensitive composition, insulating film and organic EL display element. US Patent 6797450B2
Tomikawa M, Suwa M, Yoshida S, Fujita Y, Okuda R, Ohbayashi G (2000) Novel positive-type photosensitive polyimide coatings “PW-1000”. J Photopolym Sci Technol 13:357–360
Okuda R, Miyoshi K, Arai N, Tomikawa M, Ohbayashi G (2002) Low-temperature-curing type positive-tone photosensitive polyimide coatings for insulating layer in OLED displays. J Photopolym Sci Technol 15:205–208
Okuda R, Miyoshi K, Arai N, Tomikawa M (2004) Polyimide coatings for OLED applications. J Photopolym Sci Technol 17:207–214
Aoai T, Yamanaka T, Yagihara M (1997) Structural effect of polymer and inhibitor on alkali dissolution and dissolution inhibition characteristics. J Photopolym Sci Technol 10:387–396
Miyagawa K, Naruse K, Ohnishi S, Yamaguchi K, Seko K, Numa N, Iwasawa N (2001) Study on thermal crosslinking reaction of o-naphthoquinone diazides and application to electrodeposition positive photoresist. Prog Org Coat 42:20–28
Shimada T, Kajitani M, Okamoto M, Kondo N, Katayama M, Sakihana Y, Yamamoto A, Nakata Y, Nishiki H, Shimada Y (2000) Transmission type liquid crystal display device with connecting electrode and pixel electrode connected via contact hole through interlayer insulating film and method for fabricating. US Patent 6052162
Schwab JJ, Lichtenhan JD (1998) Polyhedral oligomeric silsesquioxane (POSS)-based polymers. Appl Organomet Chem 12:707–713
Matsuda A, Sasaki T, Tatsumisago M, Minami T (2000) Micropatterning on methylsilsesquioxane–phenylsilsesquioxane thick films by the sol-gel method. J Am Ceram Soc 83:3211–3213
Abe Y, Gunji T (2004) Oligo- and polysiloxanes. Prog Polym Sci 29:149–182
Liu H, Kondo S, Tanaka R, Oku H, Unno M (2008) A spectroscopic investigation of incompletely condensed polyhedral oligomeric silsesquioxanes (POSS-mono-ol, POSS-diol and POSS-triol): Hydrogen-bonded interaction and host-guest complex. J Organomet Chem 693:1301–1308
Handke M, Handke B, Kowalewska A, Jastrzebski W (2009) New polysilsesquioxane materials of ladder-like structure. J Mol Struct 924–926:254–263
Ervithayasuporn V, Abe J, Wang X, Matsushima T, Murata H, Kawakami Y (2010) Synthesis, characterization, and OLED application of oligo(p-phenylene ethynylene)s with polyhedral oligomeric silsesquioxanes (POSS) as pendant groups. Tetrahedron 66:9348–9355
Tashiro Y, Miyazato A, Ebitani K (2015) One-pot synthesis of oligo-ladder phenylsilsesquioxanes via pyridine–silicon adduct formation at aqueous–organic liquid boundary. J Inorg Organomet Polym 25:1353–1361
Yoshida N, Bermundo JP, Ishikawa Y, Nonaka T, Uraoka Y (2018) Photosensitive polysiloxane passivation fabricated at low temperature for highly reliable amorphous InGaZnO thin-film transistors. Jpn J Appl Phys 57:090306–5
Brown Jr JF, Vogt Jr LH, Katchman A, Eustance JW, Kiser KM, Krantz KW (1960) Double chain polymers of phenylsilsesquioxane. J Am Chem Soc 82:6194–6195
Brown JF, Vogt LH, Prescott PI (1964) Preparation and characterization of the lower equilibrated phenylsilsesquioxanes. J Am Chem Soc 86:1120–1125
Inoue T, Sugiyama H, Nate K, Mizushima A (1989) Photosensitive organosilicon polymers for microlithographic application. Makromol Chem Macromol Symp 24:189–199
Sachdev HS, Whitaker JR, Sachdev KG (1993) New silicon containing positive resist and its applications for sub-half micron lithography. Microelectron Eng 21:223–226
Smaihi M, Jermoumi T, Marignan J (1995) Structural study of poly(phenylsilsesquioxane) sol-gel materials. Chem Mater 7:2293–2299
Lee E, Kimura Y (1998) Synthesis and polycondensation of a cyclic oligo(phenylsilsesquioxane) as a model reaction for the formation of poly(silsesquioxane) ladder polymer. Polym J 30:730–735
Duchateau R (2002) Incompletely condensed silsesquioxanes: versatile tools in developing silica-supported olefin polymerization catalysts. Chem Rev 102:3525–3542
Takamura N, Gunji T, Hatano H, Abe Y (1999) Preparation and properties of polysilsesquioxanes: polysilsesquioxanes and flexible thin films by acid-catalyzed controlled hydrolytic polycondensation of methyl- and vinyltrimethoxysilane. J Polym Sci A Polym Chem 37:1017–1026
Feher FJ, Terroba R, Ziller JW (1999) A new route to incompletely condensed silsesquioxanes: Base-mediated cleavage of polyhedral oligosilsesquioxanes. Chem Commun 22:2309–2310
Tashiro Y, Sekito T, Iwata T, Yokoyama D, Nonaka T (2008) Development of photosensitive silsesquioxane. Proc SPIE 7140:71402O–11
Park ES, Ro HW, Ngnyen CV, Jaff RL, Yoon DY (2008) Infrared spectroscopy study of microstructures of poly(silsesquioxane)s. Chem Mater 20:1548–1554
Loy DA, Baugher BM, Baugher CR, Schneider DA, Rahimian K (2000) Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem Mater 12:3624–3632
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Silsesquioxanes. Chem Rev 95:1409–1430
Tashiro Y, Miyazato A, Ebitani K (2015) Characterization of photosensitive composition based on oligo-ladder phenylsilsesquioxane. J Photopolym Sci Technol 28:239–245
Suwa M, Kamogawa M, Fujii M, Nishiyama T, Kozuka H (2021) Intermolecular interactions between sol-gel-derived random-structure oligomeric silsesquioxanes and a diazonaphthoquinone derivative. Jpn J Appl Phys 60:051002–9
Suwa M, Kamogawa M, Fujii M, Nishiyama T, Kozuka H (2022) Patterning characteristic control of sol-gel-derived random-structure oligomeric silsesquioxanes having a diazonaphthoquinone derivative. J Vac Sci Technol B 40:022602–12
Kolezynski A, Jastrzebski W, Szczypka W, Kowalewska A, Nowacka M, Sitarz M (2013) The structure and bonding properties of chosen phenyl ladder-like silsesquioxane clusters. J Mol Struct 1044:314–322
Unno M, Matsumoto H (2007) Synthesis of laddersiloxanes by novel stereocontrolled approach. J Organomet Chem 692:307–312
Zhang ZX, Hao J, Xie P, Zhang X, Han CC, Zhang R (2008) A well-defined ladder polyphenylsilsesquioxane (Ph-LPSQ) synthesized via a new three-step approach: monomer self-organization-lyophilization-surface-confined polycondensation. Chem Mater 20:1322–1330
Smith AL, Anderson DR (1984) Vibrational spectra of Me2SiCl2, Me3SiCl, Me3SiOSiMe3, (Me2SiO)3, (Me2SiO)4, (Me2SiO)x, and their deuterated analogs. Appl Spectrosc 38:822–834
Szczypka W, Jelen K, Andrzej K (2014) Theoretical studies of bonding properties and vibrational spectra of chosen ladder-like silsesquioxane clusters. J Mol Struct 75:599–604
Sato Y, Hayami Y, Gunji T (2022) Characterization of NMR, IR, and Raman spectra for siloxanes and silsesquioxanes: a mini review. J Sol Gel Sci Technol 104:36–52
Acknowledgements
We would like to thank the staff of Electronic and Imaging Materials Research Laboratories, Toray Industries, Inc., for cooperation in carrying out this research and Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
MS wrote the manuscript text and prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suwa, M., Fujii, M., Kamogawa, M. et al. Characteristics of transparent cured film patterns prepared from sol-gel-derived positive-type photosensitive oligomeric silsesquioxanes with random structure. J Sol-Gel Sci Technol 108, 514–527 (2023). https://doi.org/10.1007/s10971-023-06199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06199-3