Abstract
The synthesis of copper oxide nanoparticles gained considerable attraction in various fields such as biomedical, catalysis, and water purification. The current study report a simple and cost-effective green based sol-gel auto combustion method for the preparation of copper oxide nano catalyst. Lemon juice enriched with various phytochemicals has been found to be, an ideal medium for the synthesis of copper oxide nanoparticles. The decomposition of the metal-citrate-nitrate gel and formation of copper oxide nanoparticles were evaluated by the aid of TG, DTG and DTA analysis. XRD, FTIR and UV analysis confirmed the formation of copper oxide in the nano range. The surface morphology and surface features of the synthesized material were also assessed by using SEM, EDAX, TEM, XPS and BET characterization techniques. The synthesized copper oxide nanoparticles found to exhibit acceptable antibacterial efficacy against E.coli and B.subtilis. The antibacterial nature of the nanomaterial make them more suitable for biomedical applications. The catalytic activity of copper oxide nanoparticles was evaluated by performing the reduction of p-nitrophenol and degradation of rhodamine B with the assistance of NaBH4. This method provides a simple, highly economic and environmentally benign route for the synthesis of efficient copper oxide nanoparticle and overcome all the demerits of colloidal synthesis.
Graphical Abstract

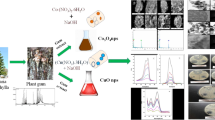
Graphical abstract describes lime juice mediated sol-gel synthesis of copper oxide nanoparticles and its excellent catalysy efficiency towards the reduction of p-nitrophenol and, antibacterial efficacy towards various harmful pathogens.













Similar content being viewed by others
Data availability
The authors confirm that the datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Oyewo OA et al. (2020) Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 5(30):18798–18807.
Buledi JA et al. (2021) Heterogeneous kinetics of CuO nanoflakes in simultaneous decolorization of Eosin Y and Rhodamine B in aqueous media. Appl Nanosci 11(4):1241–1256.
Alla SK et al. (2016) Solvothermal synthesis of CuO–MgO nanocomposite particles and their catalytic applications. RSC Adv 6(66):61927–61933.
Nezafat, Z, et al. (2021) A promising nanocatalyst: Upgraded Kraft lignin by titania and palladium nanoparticles for organic dyes reduction. Inorg Chem Commun. 130.
Bhat SA et al. (2020) Highly efficient catalytic reductive degradation of Rhodamine-B over Palladium-reduced graphene oxide nanocomposite. Chem Phys Lett 754:137724.
Varadavenkatesan T et al. (2019) Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J Photochem Photobiol B 199:111621.
Sahu K, Singhal R, Mohapatra S (2019) Morphology controlled CuO nanostructures for efficient catalytic reduction of 4-nitrophenol. Cat Lett 150(2):471–481.
Abay AK, Chen X, Kuo D-H (2017) Highly efficient noble metal free copper nickel oxysulfide nanoparticles for catalytic reduction of 4-nitrophenol, methyl blue, and rhodamine-B organic pollutants. New J Chem 41(13):5628–5638.
Ayodhya D, Veerabhadram G (2019) Facile thermal fabrication of CuO nanoparticles from Cu(II)-Schiff base complexes and its catalytic reduction of 4-nitrophenol, antioxidant, and antimicrobial studies. Chem Data Coll 23:100259.
Riaz Q et al. (2020) NiO nanoparticles for enhanced removal of methyl orange: equilibrium, kinetics, thermodynamic and desorption studies. Int J Environ Anal Chem 102(1):84–103.
Rupashree MP et al. (2021) Cost effective photocatalytic and humidity sensing performance of green tea mediated copper oxide nanoparticles. Inorg Chem Commun 134:108974.
Pisitsak P et al. (2021) Synthesis of gold nanoparticles using tannin-rich extract and coating onto cotton textiles for catalytic degradation of congo red. J Nanotechnol 2021:1–7.
Bordbar M, Sharifi-Zarchi Z, Khodadadi B (2016) Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue. J Sol-Gel Sci Technol 81(3):724–733.
Pramothkumar A et al. (2019) A comparative analysis on the dye degradation efficiency of pure, Co, Ni and Mn-doped CuO nanoparticles. J Mat Sci: Mater Electron 30(20):19043–19059.
Nabil B et al. (2018) Polyfunctional cotton fabrics with catalytic activity and antibacterial capacity. Chem Eng J 351:328–339.
Hashemi Salehi M et al. (2019) Application of palladium nanoparticle‐decorated Artemisia abrotanum extract‐modified graphene oxide for highly active catalytic reduction of methylene blue, methyl orange and rhodamine B. Appl Organometallic Chem 33(10):e5123.
Benhadria N et al. (2021) Catalytic reduction of methylene blue dye by copper oxide nanoparticles. J Cluster Sci 33(1):249–260.
Najafi M, Azizian S (2020) Catalytic reduction of 4-nitrophenol on the surface of copper/copper oxide nanoparticles: a kinetics study. Appl Nanosci 10(10):3827–3837.
Dutta A, Patra AK, Bhaumik A (2012) Porous organic–inorganic hybrid nickel phosphonate: Adsorption and catalytic applications. Microporous Mesoporous Mat 155:208–214.
Microwave Aided Synthesis of Silver and Gold Nanoparticles and their Antioxidant, Antimicrobial and Catalytic Potentials.
Alikhani N et al. (2022) Green synthesis of gold nanoparticles (Au NPs) using Rosa canina fruit extractand evaluation of its catalytic activity in the degradation of organic dye pollutants of water. Inorg Chem Commun 139:2013.
Bekru AG et al. (2021) Microwave-assisted synthesis of CuO nanoparticles using cordia africana lam. leaf extract for 4-nitrophenol reduction. J Nanotechnol 2021:1–12.
Sahu K et al. (2020) Enhanced catalytic activity of CuO/Cu2O hybrid nanowires for reduction of 4-nitrophenol in water. J Phys Chem Solids 136:109143.
Patra AK, Dutta A, Bhaumik A (2010) Cu nanorods and nanospheres and their excellent catalytic activity in chemoselective reduction of nitrobenzenes. Catal Commun 11(7):651–655.
Pantawane PK et al. (2020) Phytoreduced copper oxide nanoparticles by using Murraya koenigii leaf extract and its antibacterial activity. Mater Today: Proc 29:934–938.
Sagadevan S et al. (2019) Synthesis and evaluation of the structural, optical, and antibacterial properties of copper oxide nanoparticles. Appl Phys A 125(8):1–9.
Sathiyavimal S et al. (2021) Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J Environ Chem Eng 9(2):105033.
Dadi R et al. (2019) Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater Sci Eng C Mater Biol Appl 104:109968.
Sharma S et al. (2021) Eco-friendly Ocimum tenuiflorum green route synthesis of CuO nanoparticles: Characterizations on photocatalytic and antibacterial activities. J Environ Chem Eng 9(4):105395.
Arunkumar B, Johnson Jeyakumar S, Jothibas M (2019) A sol-gel approach to the synthesis of CuO nanoparticles using Lantana camara leaf extract and their photo catalytic activity. Optik 183:698–705.
Li Y et al. (2020) Microwave-assisted hydrothermal synthesis of copper oxide-based gas-sensitive nanostructures. Rare Metals 40(6):1477–1493.
Chen M et al. (2019) In situ preparation of well-dispersed CuO nanocatalysts in heavy oil for catalytic aquathermolysis. Petroleum Sci 16(2):439–446.
Rangel WM, Boca Santa RAA, Riella HG (2020) A facile method for synthesis of nanostructured copper (II) oxide by coprecipitation. J Mater Res Technol 9(1):994–1004.
Parashar M, Shukla VK, Singh R (2020) Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J Mater Sci: Mater Electron 31(5):3729–3749.
Esposito S (2019) “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials (Basel) 12(4):668.
Arya S et al. (2021) Review—Influence of processing parameters to control morphology and optical properties of Sol-Gel synthesized ZnO nanoparticles. ECS J Solid State Sci Technol 10(2):023002.
Malik P et al. (2014) Green chemistry based benign routes for nanoparticle synthesis. J Nanopart 2014:1–14.
Singh S et al. (2017) Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem Eng J 313:283–292.
Krishnan RR et al. (2022) A novel approach for the fabrication of Cobalt ferrite and Nickel ferrite nanoparticles—magnetic and electrocatalytic studies. J Mater Sci: Mater Electron 33(21):17100–17112.
Sharma M, Pathak M, Kapoor PN (2018) The Sol-Gel method: pathway to ultrapure and homogeneous mixed metal oxide nanoparticles. Asian J Chem 30(7):1405–1412.
Durga Prasad P, Hemalatha J (2019) Enhanced magnetic properties of highly crystalline cobalt ferrite fibers and their application as gas sensors. J Magn Magnetic Mater 484:225–233.
Sharmila G, Thirumarimurugan M, Sivakumar VM (2016) Optical, catalytic and antibacterial properties of phytofabricated CuO nanoparticles using Tecoma castanifolia leaf extract. Optik 127(19):7822–7828.
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7(1):70.
V M et al. (2022) Dielectric and electrochemical performance of rhombohedral lanthanum manganite perovskite nanostructures. New J Chem 46(41):19874–19887.
Rashad M et al. (2013) CuO and Co3O4 nanoparticles: synthesis, characterizations, and raman spectroscopy. J Nanomater 2013:1–6. p
Tenkyong T et al. (2015) Investigation of sol-gel processed CuO/SiO2 nanocomposite as a potential photoanode material. Mater Sci-Poland 33(4):826–834.
Barati MR (2009) Characterization and preparation of nanocrystalline MgCuZn ferrite powders synthesized by sol–gel auto-combustion method. J Sol-Gel Sci Technol 52(2):171–178.
Lv W et al. (2019) Molybdenum-doped CuO nanosheets on Ni foams with extraordinary specific capacitance for advanced hybrid supercapacitors. J Mater Sci 55(6):2492–2502.
Yan Q et al. (2021) Simple fabrication of bimetallic platinum-rhodium alloyed nano-multipods: A highly effective and recyclable catalyst for reduction of 4-nitrophenol and rhodamine B. J Colloid Interface Sci 582(Pt B):701–710.
Kassem AA et al. (2021) Catalytic reduction of 4-nitrophenol using copper terephthalate frameworks and CuO@C composite. J Environ Chem Eng 9(1):104401.
Sahu K, Singh J, Mohapatra S (2019) Catalytic reduction of 4-nitrophenol and photocatalytic degradation of organic pollutants in water by copper oxide nanosheets. Opt Mater 93:58–69.
Manjari G et al. (2017) Catalytic and recyclability properties of phytogenic copper oxide nanoparticles derived from Aglaia elaeagnoidea flower extract. J Saudi Chem Soc 21(5):610–618.
Nandanwar SU, Chakraborty M (2012) Synthesis of colloidal CuO/γ-Al2O3 by microemulsion and its catalytic reduction of aromatic nitro compounds. Chin J Cat 33(9-10):1532–1541.
Sasmal AK, Dutta S, Pal T (2016) A ternary Cu2O-Cu-CuO nanocomposite: a catalyst with intriguing activity. Dalton Trans 45(7):3139–3150.
Bhattacharjee A, Ahmaruzzaman M (2016) CuO nanostructures: facile synthesis and applications for enhanced photodegradation of organic compounds and reduction of p-nitrophenol from aqueous phase. RSC Adv 6(47):41348–41363.
Konar S et al. (2016) Shape-dependent catalytic activity of CuO nanostructures. J Cat 336:11–22. p
Thawarkar SR et al. (2018) Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles. RSC Adv 8(67):38384–38390.
Deka P, Deka RC, Bharali P (2014) In situ generated copper nanoparticle catalyzed reduction of 4-nitrophenol. New J Chem 38(4):1789–1793.
Jiang J et al. (2018) Hierarchical Cu nanoparticle-aggregated cages with high catalytic activity for reduction of 4-nitrophenol and carbon dioxide. Mater Res Bull 100:184–190.
Al-Ghamdi YO, Khan SA (2020) Stabilization of zero-valent Au nanoparticles on carboxymethyl cellulose layer coated on chitosan-CBV 780 zeolite Y sheets: assessment in the reduction of 4-nitrophenol and dyes. Cellulose 27(15):8827–8841.
Andualem WW et al. (2020) Synthesis of copper oxide nanoparticles using plant leaf extract of catha edulis and its antibacterial activity. J Nanotechnol 2020:1–10.
Pandiyarajan T et al. (2013) Synthesis and concentration dependent antibacterial activities of CuO nanoflakes. Mater Sci Eng C Mater Biol Appl 33(4):2020–2024.
Slavin YN et al. (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnology 15(1):65.
Acknowledgements
The authors acknowledge KSCSTE, Government of Kerala and the DST, Government of India for the instrumentation facilities provided under the SARD (No.612/2016/KSCSTE) and FIST schemes (SR/FIST/College-238/2014(c). Authors also acknowledge STIC-CUSAT Kochi for providing TEM facility. One of the author EJ acknowledge University Grant Commission Delhi (UGC) and RRK acknowledge Council for Scientific and Industrial Research (CSIR; Government of India) for providing research fellowship. The authors extend their gratitude to Dr. Sudeesh N, Assistant Professor, NSS College Cherthala for the fruitful discussion about TON and TOF.
Author contributions
EJ: Conceptualization, Investigation, Formal Analysis, and Writing original draft. RRK: Formal Analysis & Reviewing SRC: Formal analysis PKH: Resources, Conceptualization, Validation, Reviewing & Editing, Supervision, Project Administration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johnson, E., Krishnan, R.R., Chandran, S.R. et al. Green mediated sol-gel synthesis of copper oxide nanoparticle: An efficient candidate for waste water treatment and antibacterial agent. J Sol-Gel Sci Technol 107, 697–710 (2023). https://doi.org/10.1007/s10971-023-06172-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06172-0