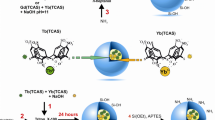
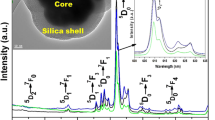
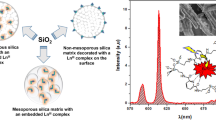
Abstract
Designing luminescent nanohybrids for bioimaging proposes has been explored by different approaches in the literature. In this context, here silica luminescent nanohybrids containing Eu3+-complexes were synthesized in three different approaches to determine the better methodology to obtain the most efficient emissive final hybrid and its applicability in cell imaging by using the Eu3+ luminescent probe properties. For this, the synthesized dense Stöber silica nanoparticles, SiO2, had their surface functionalized with APTES, in which its amine group reacted with salicylaldehyde to form a Schiff base ligand (SB), yielding the SiO2-SB system. Then, Eu3+ ion was coordinated to the SB, followed by the displacement of coordinated water molecules by dibenzoylmethane (dbm), resulting in the SiO2-[Eu1] hybrid. SiO2-[Eu2] hybrid, in turn, was obtained from tris-[Eu(dbm)3] complexes coordinated to the imine groups grafted on the SiO2-SB surface. For the third hybrid, SiO2-[Eu3], a new Eu3+-Schiff base complex displaying a triethoxysilyl group was grafted onto the SiO2 surface. The three luminescent hybrids are spheroidal shaped with 100 nm-size and they are red emitters with long lifetime (0.34–0.61 ms) and high photostability when exposed to continuous 340 nm UV radiation. Quantum efficiency (\(Q_{{\rm{Eu}}}^{{\rm{Eu}}}\)) as well as the number of coordinated water molecules (qH2O) to the Eu3+ was estimated using the LUMPAC software package and Horrocks equation, respectively. Although the three strategies exhibited suitable photophysical results, SiO2-[Eu1] was classified as the best hybrid considering its higher \(Q_{{\rm{Eu}}}^{{\rm{Eu}}}\) and color purity values, and it was evaluated as non-toxic according to its bio-viability in CHO-k1 cells in different doses. Exploratory cell imaging tests using such hybrid indicated cell marking near the nucleus with the internalization of nanoparticles in the cell confirmed by Eu3+ (5D0 → 7FJ) narrow emission bands. Therefore, SiO2-[Eu1] hybrid manifested suitable shape and size, optical, and biocompatibility features that make it promising to be applied as a luminescent stain for cell imaging.
Graphical abstract

Left—Graph expressing the cell viability from the cytotoxicity test of CHO-k1 cells as function of concentration performed with the hybrids SiO2-[Eu1], SiO2-[Eu2], and SiO2-[Eu3], obtained from Approach 1, Approach 2, and Approach 3, respectively. Middle—Suggested final structures of complexes grafted on silica particles for the three tested approaches. Right—TEM, and Confocal images of CHO-k1 cells incubated with SiO2-[Eu1.
Highlights
-
Three different approaches were evaluated to prepair luminescent hybrids containing Eu3+-complexes.
-
Three spherical 100 nm-sized hybrids with long lifetimes and emitting red were produced.
-
Three spherical 100 nm-sized hybrids with long lifetimes and emitting red were produced.








Similar content being viewed by others
References
Eliseeva SV, Bünzli JCG (2010) Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev 39(1):189–227. https://doi.org/10.1039/B905604C
Silva CL, Bispo-Jr AG, Lima SAM, Pires AM (2019) Eu3+ complex/polymer films for light-emitting diode applications. Opt Mater 96:109323. https://doi.org/10.1016/j.optmat.2019.109323
Leite Silva CM, Bispo‐Jr AG, Canisares FS, Castilho SA, Lima SA, Pires AM (2019) Eu3 + ‐tetrakis β‐diketonate complexes for solid‐state lighting application. Luminescence 34(8):877–886. https://doi.org/10.1002/bio.3686
Bispo AG, Lima SA, Carlos LD, Ferreira RA, Pires AM (2019) Red-emitting coatings for multifunctional UV/Red emitting LEDs applied in plant circadian rhythm control. ECS J Solid State Sci Technol 9(1):016008, https://iopscience.iop.org/article/10.1149/2.0122001JSS/meta
Bünzli JCG (2010) Lanthanide luminescence for biomedical analyses and imaging. Chem Rev 110(5):2729–2755. https://doi.org/10.1021/cr900362e
Wang F, Tan WB, Zhang Y, Fan X, Wang M (2005) Luminescent nanomaterials for biological labelling. Nanotechnology 17(1):R1. https://iopscience.iop.org/article/10.1088/0957-4484/17/1/R01/meta
Enrichi F, Trave E, Bersani M (2008) Acid synthesis of luminescent amine-functionalized or erbium-doped silica spheres for biological applications. J Fluoresc 18:507–511. https://doi.org/10.1007/s10895-007-0292-z
Bünzli JCG, Eliseeva SV (2011) Basics of lanthanide photophysics. In: Hänninen, P., Härmä, H. (eds) Lanthanide Luminescence. Springer Series on Fluorescence, vol 7. Springer, Berlin, Heidelberg. https://doi.org/10.1007/4243_2010_3
Allendorf MD, Bauer CA, Bhakta RK, Houk RJT (2009) Luminescent metal–organic frameworks. Chem Soc Rev 38(5):1330–1352. https://doi.org/10.1039/B802352M
Karooby E, Granpayeh N (2019) Potential applications of nanoshell bow-tie antennas for biological imaging and hyperthermia therapy. Opt Eng 58(6):065102. https://doi.org/10.1117/1.OE.58.6.065102
Wang Y, Chang H, Jia L, Zhu T, Xu Z, Zhou T, Li H, Li Z, Xu J (2015) Development of a visible-light-sensitized THA-based lanthanide nanocomposite for cell imaging. Mater Lett 161:644–647. https://doi.org/10.1016/j.matlet.2015.09.073
Zhao Q, Liu Y, Cao Y, Lv W, Yu Q, Liu S, Liu X, Shi M, Huang W (2015) Rational design of nanoparticles with efficient lanthanide luminescence sensitized by iridium (III) complex for time‐gated luminescence bioimaging. Adv Opt Mater 3(2):233–240. https://doi.org/10.1002/adom.201400464
Jaque D, Richard C, Viana B, Soga K, Liu X, Sole JG (2016) Inorganic nanoparticles for optical bioimaging. Adv Opt Photonics 8(1):1–103. https://doi.org/10.1364/AOP.8.000001
Tang L, Cheng J (2013) Nonporous silica nanoparticles for nanomedicine application. Nano Today 8(3):290–312. https://doi.org/10.1016/j.nantod.2013.04.007
Wang L, Zhao W, Tan W (2008) Bioconjugated silica nanoparticles: development and applications. Nano Res 1:99–115. https://doi.org/10.1007/s12274-008-8018-3
Liberman A, Mendez N, Trogler WC, Kummel AC (2014) Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf Sci Rep 69(2-3):132–158. https://doi.org/10.1016/j.surfrep.2014.07.001
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69. https://doi.org/10.1016/0021-9797(68)90272-5
Gonçalves MC (2018) Sol-gel silica nanoparticles in medicine: a natural choice. Design, synthesis and products. Molecules 23(8):2021. https://doi.org/10.3390/molecules23082021
Chen L, Liu J, Zhang Y, Zhang G, Kang Y, Chen A, Feng X, Shao L (2018) The toxicity of silica nanoparticles to the immune system. Nanomedicine 13(15):1939–1962. https://doi.org/10.2217/nnm-2018-0076
Croissant JG, Butler KS, Zink JI, Brinker CJ (2020) Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat Rev Mater 5(12):886–909. https://doi.org/10.1038/s41578-020-0230-0
Kim IY, Joachim E, Choi H, Kim K (2015) Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed Nanotechnol Biol Med 11(6):1407–1416. https://doi.org/10.1016/j.nano.2015.03.004
Duarte AP, Gressier M, Menu MJ, Dexpert-Ghys J, Caiut JMA, Ribeiro SJ (2012) Structural and luminescence properties of silica-based hybrids containing new silylated-diketonato europium (III) complex. J Phys Chem C 116(1):505–515. https://doi.org/10.1021/jp210338t
Lourenço AVS, Kodaira CA, Ramos-Sanchez EM, Felinto MCF, Goto H, Gidlund M, Malta OL, Brito HF (2013) Luminescent material based on the [Eu (TTA) 3 (H2O) 2] complex incorporated into modified silica particles for biological applications. J Inorg Biochem 123:11–17. https://doi.org/10.1016/j.jinorgbio.2013.02.006
Li QP, Yan B (2014) Luminescent nanoparticles prepared by encapsulating lanthanide chelates to silica sphere. Colloid Polym Sci 292:1385–1393. https://doi.org/10.1007/s00396-014-3196-x
Ilibi M, de Queiroz TB, Ren J, De Cola L, de Camargo ASS, Eckert H (2014) Luminescent hybrid materials based on covalent attachment of Eu (III)-tris (bipyridinedicarboxylate) in the mesoporous silica host MCM-41. Dalton Trans 43(22):8318–8330. https://doi.org/10.1039/C3DT52096J
Mutti AMG, Santos JAO, Cavalcante DGSM, Gomes AS, Job AE, Teixeira GR, Lima SAM (2019) Design of a red-emitter hybrid material for bioimaging: europium complexes grafted on silica particles. Mater Today Chem 14:100204. https://doi.org/10.1016/j.mtchem.2019.100204
Mutti AMG, Santos JAO, Cavalcante DGSM, Gomes AS, Job AE, Pires AM, Lima SAM (2019) Decorated silica particles with terbium complexes as luminescent biomarker for cell imaging. Opt Mater 90:57–63. https://doi.org/10.1016/j.optmat.2019.02.019
Santos JA, Mutti AM, Bispo-Jr AG, Pires AM, Lima SA (2020) Red-emitting hybrid based on Eu3 + -dbm complex anchored on silica nanoparticles surface by carboxylic acid for biomarker application. Materials 13(23):5494. https://doi.org/10.3390/ma13235494
Binnemans K (2015) Interpretation of europium (III) spectra. Coord Chem Rev 295:1–45. https://doi.org/10.1016/j.ccr.2015.02.015
Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams G, Woods M (1999) Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states. J Chem Soc Perkin Trans 2 3:493–504. https://doi.org/10.1039/A808692C
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Sing KS(1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619. https://doi.org/10.1351/pac198557040603
Gelamos JP, Laranja ML, Alvino KCL, Camacho SA, Pires AM (2009) Up-converter nanophosphor Y2O2S: Er, Yb aminofunctionalized containing or not spherical silica conjugated with BSA. J Lumin 129(12):1726–1730. https://doi.org/10.1016/j.jlumin.2009.04.029
Allen LH, Matijevíc E, Meites L (1971) Exchange of Na+ for the silanolic protons of silica. J Inorg Nucl Chem 33(5):1293–1299. https://doi.org/10.1016/0022-1902(71)80423-2
Lide DR (2004) CRC Handbook of Chemistry and Physics, 85th ed. vol 85. CRC Press: Boca Raton, FL, p 3–150
de Oliveira LF, Bouchmella K, Goncalves KDA, Bettini J, Kobarg J, Cardoso MB (2016) Functionalized silica nanoparticles as an alternative platform for targeted drug-delivery of water insoluble drugs. Langmuir 32(13):3217–3225. https://doi.org/10.1021/acs.langmuir.6b00214
Huang CH (2010) Rare Earth Coordination Chemistry: Fundamentals and Applications. John Wiley & Sons (Asia) Pte Ltd: Singapore.
Binnemans K (2009) Lanthanide-based luminescent hybrid materials. Chem Rev 109(9):4283–4374. https://doi.org/10.1021/cr8003983
Santa-Cruz PA, Teles FS (2003) Spectra Lux Software v.2.0, Ponto Quântico Nanodispositivos
Horrocks Jr WD, Sudnick DR (1981) Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc Chem Res 14(12):384–392. https://doi.org/10.1021/ar00072a004
Supkowski RM, Horrocks Jr WD (2002) On the determination of the number of water molecules, q, coordinated to europium (III) ions in solution from luminescence decay lifetimes. Inorg Chim Acta 340:44–48. https://doi.org/10.1016/S0020-1693(02)01022-8
Judd BR (1962) Optical absorption intensities of rare-earth ions. Phys Rev 127(3):750. https://doi.org/10.1103/PhysRev.127.750
Ofelt GS (1962) Intensities of crystal spectra of rare‐earth ions. J Chem Phys 37(3):511–520. https://doi.org/10.1063/1.1701366
Filho MA, Dutra JDL, Cavalcanti HL, Rocha GB, Simas AM, Freire RO (2014) RM1 model for the prediction of geometries of complexes of the trications of Eu, Gd, and Tb. J Chem Theory Comput 10(8):3031–3037. https://doi.org/10.1021/ct400909w
Dutra JDL, Bispo TD, Freire RO (2014) LUMPAC lanthanide luminescence software: efficient and user friendly. J Comput Chem 35(10):772–775. https://doi.org/10.1002/jcc.23542
Silva AI, Santos VF, Lima NB, Simas AM, Gonçalves SM (2016) Substantial luminescence enhancement in ternary europium complexes by coordination of different ionic ligands. RSC Adv 6(93):90934–90943. https://doi.org/10.1039/C6RA20609C
Duarte AP, Mauline L, Gressier M, Dexpert-Ghys J, Roques C, Caiut JMA, Deffune E, Maia DCG, Carlos IZ, Ferreira AAP, Ribeiro SJL, Menu MJ (2013) Organosilylated complex [Eu (TTA) 3 (Bpy-Si)]: a bifunctional moiety for the engeneering of luminescent silica-based nanoparticles for bioimaging. Langmuir 29(19):5878–5888. https://doi.org/10.1021/la400365c
Bünzli JCG (2016) Lanthanide light for biology and medical diagnosis. J Lumin 170:866–878. https://doi.org/10.1016/j.jlumin.2015.07.033
Martinić I, Eliseeva SV, Petoud S (2017) Near-infrared emitting probes for biological imaging: organic fluorophores, quantum dots, fluorescent proteins, lanthanide (III) complexes and nanomaterials. J Lumin 189:19–43. https://doi.org/10.1016/j.jlumin.2016.09.058
Chen L, Liu J, Zhang Y, Zhang G, Kang Y, Chen A, Feng X, Shao L (2018) The toxicity of silica nanoparticles to the immune system. Nanomedicine 13(15):1939–1962. https://doi.org/10.2217/nnm-2018-0076
Mohammadi P, Abbasinia M, Assari MJ, Oliaei M (2018) The toxicology of silica nanoparticles: a review. Toxicol Environ Chem 100(3):285–316. https://doi.org/10.1080/02772248.2018.1485921
BS EN ISO STANDARD B (2010) Biological evaluation of medical devices. Part 5: tests for in vitro cytotoxicity
Singh NP et al. (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Dworetzky SI et al. (1988) The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol 107:1279–1287. https://doi.org/10.1083/jcb.107.4.1279
Kim IY, Joachim E, Choi H, Kim K (2015) Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed Nanotechnol Biol Med 11(6):1407–1416. https://doi.org/10.1016/j.nano.2015.03.004
Soenen SJ, Manshian B, Doak SH, De Smedt SC, Braeckmans K (2013) Fluorescent non-porous silica nanoparticles for long-term cell monitoring: cytotoxicity and particle functionality. Acta Biomater 9(11):9183–9193. https://doi.org/10.1016/j.actbio.2013.04.026
Hashemi E, Akhavan O, Shamsara M, Rahighi R, Esfandiar A, Tayefeh AR (2014) Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv 4:27213–27223. https://doi.org/10.1039/c4ra01047g
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5:6971–6980. https://doi.org/10.1021/nn202451x
Krętowski R, Kusaczuk M, Naumowicz M, Kotyńska J, Szynaka B, Cechowska-Pasko M (2017) The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials 7:230. https://doi.org/10.3390/nano7080230.
Monteiro JHSK, Machado D, De Hollanda LM, Lancellotti M, Sigoli FA, de Bettencourt-Dias A (2017) Selective cytotoxicity and luminescence imaging of cancer cells with a dipicolinato-based Eu III complex. Chem Commun 53(86):11818–11821. https://doi.org/10.1039/C7CC06753D
Qiu K, Zhu H, Rees TW, Ji L, Zhang Q, Chao H (2019) Recent advances in lysosome-targeting luminescent transition metal complexes. Coord Chem Rev 398:113010. https://doi.org/10.1016/j.ccr.2019.07.007
Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC (2000) Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci 113(9):1515–1524. https://doi.org/10.1242/jcs.113.9.1515
Schneider CA, Rasband WS, Eliceiri KW(2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Acknowledgements
The authors thank FAPESP (2019/26103-7) for the financial research support, and also for the scholarships AMGM (CAPES 1531833), FSMC (CAPES 88887.341772/2019-00), JAOS (CNPq 141229/2019-5), BCS (PIBIC/Reitoria), AMP (CNPq 304003/2018-1), and SAML (CNPq 308868/2022-6). Scanning Electron Microscopy Multiuser Laboratory (LabMMEV/rof. Dr. Neri Alves, Department of Physics, FCT-UNESP); Nitrogen Adsorption/Desorption Isotherms (Analytical Center—Chemistry Institute UNICAMP, Prof. Dr. Heloíse Pastore); Vibrational Absorption Spectroscopy in the Infrared region with Fourier Transform FTIR (Central Laboratory of the Department of Chemistry and Biochemistry—FCT-UNESP); Elemental Analysis (Multi Usual Experimental Centers at the Federal University of ABC—Prof. Dr. Karina Frinn); Zeta Potential (Laboratory of Magnetic Materials and Colloids—LaMMC, Department of Physical Chemistry of the Institute of Chemistry—UNESP Araraquara Campus, Prof. Dr. Rodrigo Fernando Costa Marques, and Prof. Dr. Miguel Jafelicci Junior) and (Nanostructured Materials Laboratory for Environmental and Biological Analysis—Department of Physics, FCT-UNESP—Prof. Dr. Carlos José Leopoldo Constantino); Photoluminescence Spectroscopy (Sol–Gel Research Group—UNIFRAN, Prof. Dr. Eduardo José Nassar); Fluorescence and Confocal Microscopy (Nanostructured Materials Laboratory for Environmental and Biological Analysis—Department of Physics, FCT-UNESP—Prof. Dr. Carlos José Leopoldo Constantino); Transmission Electron Microscopy (Electron Microscopy Laboratory—LME, Chemistry Institute of USP São Carlos, under the supervision of Ana Curro).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mutti, A.M.G., Canisares, F.S.M., Santos, J.A.O. et al. Silica-based nanohybrids containing europium complexes covalently grafted: structural, luminescent, and cell labeling investigation. J Sol-Gel Sci Technol 107, 754–770 (2023). https://doi.org/10.1007/s10971-023-06138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06138-2