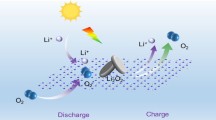
Abstract
This paper studies how electrochemically inserting or extracting lithium ions in TiO2 can trigger and orient solar hydrogen evolution reaction. LixTiO2 is envisioned as a photo(electro)catalyst, whose rejuvenation can be achieved by in situ potential control. The investigations are conducted in a half-battery cell design where a mesoporous, anatase TiO2 thin film (negative electrode candidate) is deposited onto a transparent conducting oxide (FTO) substrate, water-in-salt (WiSE) is used as an electrolyte. Changing the state of charge of the working electrode material, will adjust the ratio between the Li-rich phase (Li0.5TiO2 blue) and the Li-poor phase (Li0.01TiO2 uncolored), which will tune the opto-electronic properties of the material, potentially changing the light-matter interaction. The impact of light on the interplay between hydrogen evolution reaction and lithium insertion/extraction in TiO2 is investigated using classical electrochemical characterizations inspired from both battery and photoelectrode communities. With a minimum of Li-rich phase solar hydrogen is produced at the working or at the counter electrode while lithium in photo-extracted of the Li-rich phase to reform TiO2.
Graphical Abstract

This paper deals with the interplay between hydrogen evolution reaction and lithium insertion/extraction in TiO2 under light. To investigate this behavior, mesoporous, crystalline TiO2 thin films, synthesized by the sol-gel chemistry, have been investigated as working electrodes of a Li-ion battery to control in situ its chemical composition by inserting Li+ to study the photo(electro)catalytical decomposition of water in WiSE.
Highlights
-
Triggering the HER via a control of potential.
-
Understanding the interplay between hydrogen evolution reaction and lithium insertion/extraction in TiO2 under light.
-
Li-rich phase is photo(electro)chemical active in the visible range.
-
Controlling in space, the localization of the HER.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Droguet L, Grimaud A, Fontaine O, Tarascon J-M (2020) Water-in-Salt Electrolyte (WiSE) for Aqueous Batteries: A Long Way to Practicality. Adv Energy Mater 10:2002440. https://doi.org/10.1002/aenm.202002440
Sui Y, Ji X (2021) Anticatalytic strategies to suppress water electrolysis in aqueous batteries. Chem Rev 121:6654–6695. https://doi.org/10.1021/acs.chemrev.1c00191
Zeng Q et al. (2020) Integrated photorechargeable energy storage system: Next-Generation power source driving the future. Adv Energy Mater 10:1903930. https://doi.org/10.1002/aenm.201903930
Rodríguez-Seco C, Wang Y-S, Zaghib K, Ma D (2022) Photoactive nanomaterials enabled integrated photo-rechargeable batteries. Nanophotonics 11:1443–1484. https://doi.org/10.1515/nanoph-2021-0782
Shimokawa K, Matsubara S, Okamoto A, Ichitsubo T (2022) Light-induced Li extraction from LiMn2O4/TiO2 in a water-in-salt electrolyte for photo-rechargeable batteries. Chem Commun 58:9634–9637. https://doi.org/10.1039/D2CC03362C
Cao L, Skyllas-Kazacos M, Wang D-W (2018) Solar redox flow batteries: Mechanism, design, and measurement. Adv Sustain Syst 2:1800031. https://doi.org/10.1002/adsu.201800031
Lei B, Li G, Chen P, Gao X (2017) A solar rechargeable battery based on hydrogen storage mechanism in dual-phase electrolyte. Nano Energy 38:257–262. https://doi.org/10.1016/j.nanoen.2017.06.001
Li N, Wang Y, Tang D, Zhou H (2015) Integrating a photocatalyst into a hybrid Lithium-Sulfur battery for direct storage of solar energy. Angew Chem (Int ed Engl) 54:9271–9274. https://doi.org/10.1002/anie.201503425
Sood A et al. (2021) Electrochemical ion insertion from the atomic to the device scale. Nat Rev Mater 6:847–867. https://doi.org/10.1038/s41578-021-00314-y
Hu Y et al. (2020) Lattice distortion induced internal electric field in TiO(2) photoelectrode for efficient charge separation and transfer. Nat Commun 11:2129. https://doi.org/10.1038/s41467-020-15993-4
Lu Y et al. (2018) Self-hydrogenated shell promoting photocatalytic H(2) evolution on anatase TiO(2). Nat Commun 9:2752. https://doi.org/10.1038/s41467-018-05144-1
Sum J, Durupthy O, Krins N, Laberty-Robert C (2022) Regeneration of electrocatalyst through Li-Ion insertion. J Electrochem Soc 169:030522. https://doi.org/10.1149/1945-7111/ac5ad4
Makivić N et al. (2021) Evidence of bulk proton insertion in nanostructured anatase and Amorphous TiO2 electrodes. Chem Mater 33:3436–3448. https://doi.org/10.1021/acs.chemmater.1c00840
Kim Y-S et al. (2017) Evidencing fast, massive, and reversible H+ insertion in nanostructured TiO2 electrodes at neutral pH. Where do protons come from? J Phys Chem C 121:10325–10335. https://doi.org/10.1021/acs.jpcc.7b02395
Dahlman CJ et al. (2021) Dynamics of lithium insertion in electrochromic titanium dioxide nanocrystal ensembles. J Am Chem Soc 143:8278–8294. https://doi.org/10.1021/jacs.0c10628
Dahlman CJ, Tan Y, Marcus MA, Milliron DJ (2015) Spectroelectrochemical signatures of capacitive charging and ion insertion in doped anatase Titania nanocrystals. J Am Chem Soc 137:9160–9166. https://doi.org/10.1021/jacs.5b04933
Cha G, Ozkan S, Hwang I, Mazare A, Schmuki P (2022) Li+ doped anodic TiO2 nanotubes for enhanced efficiency of Dye-sensitized solar cells. Surf Sci 718:122012. https://doi.org/10.1016/j.susc.2021.122012
Borghols WJH et al. (2009) The electronic structure and ionic diffusion of nanoscale LiTiO2 anatase. Phys Chem Chem Phys 11:5742–5748. https://doi.org/10.1039/B823142G
Wagemaker M, Borghols WJH, Mulder FM (2007) Large impact of particle size on insertion reactions. A case for anatase Li(x)TiO2. J Am Chem Soc 129(14):4323–4327. https://doi.org/10.1021/ja067733
de Klerk NJJ, Vasileiadis A, Smith RB, Bazant MZ, Wagemaker M(2017) Explaining key properties of lithiation in \({\mathrm{TiO}}_{2}\)-anatase Li-ion battery electrodes using phase-field modeling. Phys Rev Mater. 1:025404. https://doi.org/10.1103/PhysRevMaterials.1.025404
Xia T, Zhang W, Murowchick J, Liu G, Chen X (2013) Built-in electric field-assisted surface-amorphized nanocrystals for high-rate lithium-ion battery. Nano Lett 13:5289–5296. https://doi.org/10.1021/nl402810d
Li H et al. (2022) Enhancement of Plasmon-Induced Photoelectrocatalytic Water Oxidation over Au/TiO2 with Lithium Intercalation. Angew Chem Int Ed 61:e202204272. https://doi.org/10.1002/anie.202204272
Luo Y, Li P, Jin Z (2022) Lithiated interface of Pt/TiO2 enables an efficient wire-shaped Zn–Air solar micro-battery. Chem Commun 58:5988–5991. https://doi.org/10.1039/D2CC01875F
Meekins BH, Kamat PV (2009) Got TiO2 nanotubes? Lithium ion intercalation can boost their photoelectrochemical performance. ACS nano 3:3437–3446. https://doi.org/10.1021/nn900897r
Nguyen O et al. (2017) Shedding light on the light-driven lithium ion de-insertion reaction: towards the design of a photo-rechargeable battery. J Mater Chem A 5:5927–5933. https://doi.org/10.1039/C7TA00493A
Sauvage F, Bouteau G (2020) Stark-Field Effect in Nanocrystalline Anatase TiO2 ruling miscibility gap and electrochemical performances of carbon-free electrodes for batteries. ACS Appl Energy Mater 3:8706–8715. https://doi.org/10.1021/acsaem.0c01242
Andriamiadamanana C et al. (2018) Light-induced charge separation in mixed electronic/Ionic semiconductor driving lithium-ion transfer for photo-rechargeable electrode. Adv Sustain Syst 2:1700166. https://doi.org/10.1002/adsu.201700166
Hankin A, Bedoya-Lora FE, Alexander JC, Regoutz A, Kelsall GH (2019) Flat band potential determination: Avoiding the pitfalls. J Mater Chem A 7:26162–26176. https://doi.org/10.1039/C9TA09569A
McCann JF, Pezy JP (1981) The Measurement of the Flatband Potentials of n‐Type and p‐Type semiconductors by rectified alternating photocurrent voltammetry. J Electrochem Soc 128:1735–1740. https://doi.org/10.1149/1.2127721
Acknowledgements
JS thanks the ED397 from Sorbonne University for his Ph.D. grant.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sum, J., Krins, N. & Laberty-Robert, C. Li-ion insertion coupled solar HER. J Sol-Gel Sci Technol 107, 278–288 (2023). https://doi.org/10.1007/s10971-023-06122-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06122-w