Abstract
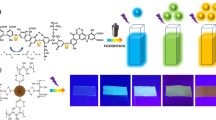
Transparent organic-inorganic SiO2-PMMA nanocomposites with different fluorescent carbon dots (CD) and rhodamine B (RB) volume ratios were obtained. Their structural and photoluminescent properties were analyzed. FT-IR characterization reveals the presence of peaks associated with Si-OH and Si-O-Si from the silica inorganic network and peaks for C=C and C=O from the organic network given by poly methyl methacrylate (PMMA) and 3-trimethoxysilylpropyl methacrylate (TMSPM) coupling agent. Deconvolution analysis of the intrinsic luminescence of the hybrid matrix and the single embedded CD and RB hybrids was performed. The nanocomposite xerogels emission spectra show two emission peaks at 440 nm and 560 nm attributed to the CD and RB, respectively. The intensity of the emission bands is related to the variation in the CD/dye concentration ratio. Also, the estimated CIE 1931 chromaticity coordinates show that the global emission tonality of nanocomposites varies from blue to yellowish-orange. The CD/dye 2:2 volume ratio sample shows an emission tonality close to the white region (x = 0.35, y = 0.33). The low manufacturing cost of these nanocomposites and the incorporation of molecules with good luminescent intensity allow their potential application as coatings in solid-state lighting devices.
Graphical Abstract

Highlights
-
Obtention of a transparent organic-inorganic nanocomposite by sol-gel process.
-
Two emission peaks changing intensity with different CD/dye concentration ratio.
-
Chromaticity coordinates near the white region (x = 0.35, y = 0.33).












Similar content being viewed by others
References
Li X et al. (2022) Synthesis of carbon dots with strong luminescence in both dispersed and aggregated states by tailoring sulfur doping. J Colloid Interface Sci 609:54–64. https://doi.org/10.1016/j.jcis.2021.11.179
Bhattacharya D, Mishra MK, De G (2017) Carbon dots from a single source exhibiting tunable luminescent colors through the modification of surface functional groups in ORMOSIL films. J Phys Chem C 121(50):28106–28116. https://doi.org/10.1021/acs.jpcc.7b08039
Schneider J et al. (2017) Molecular fluorescence in citric acid-based carbon dots. J Phys Chem C 121(3):2014–2022. https://doi.org/10.1021/acs.jpcc.6b12519
Wang Z et al. (2020) One-pot synthesis of carbon dots@ZrO2 nanoparticles with tunable solid-state fluorescence. Polym Adv Technol 31(8):1744–1751. https://doi.org/10.1002/pat.4901
Malfatti L, Innocenzi P (2018) Sol-gel chemistry for carbon dots. Chem Rec 18(7):1192–1202. https://doi.org/10.1002/tcr.201700108
Xiong Y, Schneider J, Ushakova EV, Rogach AL (2018) Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 23:124–139. https://doi.org/10.1016/j.nantod.2018.10.010
Stefania M et al. (2020) Integrating sol-gel and carbon dots chemistry for the fabrication of fluorescent hybrid organic- inorganic films. Sci Rep 1–12, https://doi.org/10.1038/s41598-020-61517-x
Khan S, Sharma A, Ghoshal S, Jain S, Hazra MK, Nandi CK (2017) Small molecular organic nanocrystals resemble carbon nanodots in terms of their properties. Chem Sci 9(1):175–180. https://doi.org/10.1039/c7sc02528a
Song Y et al. (2015) Investigation from chemical structure to photoluminescent mechanism: a type of carbon dots from the pyrolysis of citric acid and an amine. J Mater Chem C 3(23):5976–5984. https://doi.org/10.1039/c5tc00813a
Zhu S et al. (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52(14):3953–3957. https://doi.org/10.1002/anie.201300519
Zhu S, Zhao X, Song Y, Lu S, Yang B (2016) Beyond bottom-up carbon nanodots: citric-acid derived organic molecules. Nano Today 11(2):128–132. https://doi.org/10.1016/j.nantod.2015.09.002. pp
Zhou D et al. (2017) Conquering aggregation-induced solid-state luminescence quenching of carbon dots through a carbon dots-triggered silica gelation process. Chem Mater 29(4):1779–1787. https://doi.org/10.1021/acs.chemmater.6b05375
Ren J et al. (2018) Controllable photoluminescent and nonlinear optical properties of polymerizable carbon dots and their arbitrary copolymerized gel glasses. Adv Opt Mater 6(12):1–7. https://doi.org/10.1002/adom.201701273
Suzuki K et al. (2017) Design of carbon dots photoluminescence through organo-functional silane grafting for solid-state emitting devices. Sci Rep. 7(no. 1):1–11. https://doi.org/10.1038/s41598-017-05540-5
Suzuki K, Nabata H, Ueno S, Morita S, Miyamura H, Balachandran J (2022) Origin of carbon dot fluorescence in organosilica films explored experimentally by surface functionalization. J Sol Gel Sci Technol 702–710, https://doi.org/10.1007/s10971-022-05901-1
Chao T et al. (2022) Defects and structural limitation-induced carbon dots-silica hybrid materials with ultralong room temperature phosphorescence. J Phys Chem Lett 13(41):9558–9563. https://doi.org/10.1021/acs.jpclett.2c02647
Cheng H et al. (2022) Ultra-stable dual-color phosphorescence Carbon-Dot@Silica material for advanced anti-counterfeiting. Dye Pigment 208:110827. https://doi.org/10.1016/j.dyepig.2022.110827
Fang M, Neto ANC, Fu L, Ferreira RAS, deZeaBermudez V, Carlos LD (2022) A hybrid materials approach for fabricating efficient WLEDs based on di-ureasils doped with carbon dots and a europium complex. Adv Mater Technol 7(3):1–12. https://doi.org/10.1002/admt.202100727
Zhang G, Bai Y, Yu C, Xuan T (2022) Highly stable carbon nanodot-based phosphor as a color converter for WLED. J Lumin 246:118836. https://doi.org/10.1016/j.jlumin.2022.118836
Zhang H, Zhang H, Pan A, Yang B, He L, Wu Y (2021) Rare earth-free luminescent materials for WLEDs: recent progress and perspectives. Adv Mater Technol 6(1):1–26. https://doi.org/10.1002/admt.202000648
Davi LBO, Lima DJP, Barbosa CDAES (2021) Synthesis and modulation of multicolor fluorescent carbon dots from p-phenylenediamine and dansyl derivative for white light emitting diodes. Opt Mater 121:111502. https://doi.org/10.1016/j.optmat.2021.111502
Wang Z et al. (2017) Warm-white-light-emitting diode based on a dye-loaded metal-organic framework for fast white-light communication. ACS Appl Mater Interfaces 9(40):35253–35259. https://doi.org/10.1021/acsami.7b11277
Zhang Y, Ding Z, Liu Y, Zhang Y, Jiang S (2021) White-light-emitting hydrogels with self-healing properties and adjustable emission colors. J Colloid Interface Sci 582:825–833. https://doi.org/10.1016/j.jcis.2020.08.080
Tilioua A (2021) Materials Science for Energy Technologies Investigation of the thermo-physical properties of poly (methyl methacrylate) -based Plexiglass to improve the performance of solar cells. Mater Sci Energy Technol 4:349–356. https://doi.org/10.1016/j.mset.2021.08.011
Almaral-Sánchez JL, Rubio E, Mendoza-Galván A, Ramírez-Bon R (2005) Red colored transparent PMMA-SiO2 hybrid films. J Phys Chem Solids 66(10):1660–1667. https://doi.org/10.1016/j.jpcs.2005.06.006
Avnir D (1984) The nature of the silica cage as reflected by spectral changes and enhanced photostability of trapped rhodamine 6G. J Phys Chem 5959(1982):5956–5959
Costela A, García-Moreno I, Sastre R (2001) Chapter 4 - Materials for solid-state dye lasers. In Singh Nalwa, H. (ed) Handbook of Advanced Electronic and Photonic Materials and Devices 161–208. Academic Press, Los Angeles
Green WH, Le KP, Grey J, Au TT, Sailor MJ (1997) White phosphors from a silicate-carboxylate sol-gel precursor that lack metal activator ions. Science 276(5320):1826–1828. https://doi.org/10.1126/science.276.5320.1826
Amato F et al. (2019) Nitrogen-doped carbon nanodots/PMMA nanocomposites for solar cells applications. Chem Eng Trans 74:1105–1110. https://doi.org/10.3303/CET1974185
Mondal T, Bose S, Husain A, Ghorai UK, Saha SK (2020) White light emission from single dye incorporated metal organic framework. Opt Mater 100:109706. https://doi.org/10.1016/j.optmat.2020.109706
Joseph J, Anappara AA (2016) White light emission of carbon dots by creating different emissive traps. J Lumin 178:128–133. https://doi.org/10.1016/j.jlumin.2016.05.051
Lu Y, Wang S, Yu K, Yu J, Zhao D, Li C (2021) Microporous and Mesoporous Materials Encapsulating carbon quantum dot and organic dye in multi-shell nanostructured MOFs for use in white light-emitting diode. Microporous Mesoporous Mater 319:111062. https://doi.org/10.1016/j.micromeso.2021.111062
Chmel A, Mazurina EK, Shashkin VS (1990) Vibrational spectra and defect structure of silica prepared by non-organic sol-gel process. J Non Cryst Solids 122:285–290
Fidalgo A, Ilharco LM (2004) Correlation between physical properties and structure of silica xerogels. J Non Cryst Solids 347(1–3):128–137. https://doi.org/10.1016/j.jnoncrysol.2004.07.059
Gallis S, Nikas V, Kaloyeros AE (2017) Silicon oxycarbide thin films and nanostructures: synthesis, properties and applications. IntechOpen, London.
Melgoza-Ramírez ML, Ramírez-Bon R (2020) Microstructural comparison between PMMA-SiO2 and PMMA-TiO2 hybrid systems using Eu3+ as ion-probe luminescence. J Non Cryst Solids 544:120167. https://doi.org/10.1016/j.jnoncrysol.2020.120167
Soni G, Srivastava S, Soni P, Kalotra P, Vijay YK (2018) Optical, mechanical and structural properties of PMMA/SiO2 nanocomposite thin films. Mater Res Express 5(1), https://doi.org/10.1088/2053-1591/aaa0f7
Huang ZH, Qiu KY (1997) The effects of interactions on the properties of acrylic polymers/silica hybrid materials prepared by the in situ sol-gel process. Polym 38(3):521–526. https://doi.org/10.1016/S0032-3861(96)00561-7
Han YH, Taylor A, Mantle MD, Knowles KM (2007) Sol-gel-derived organic-inorganic hybrid materials. J Non Cryst Solids 353(3):313–320. https://doi.org/10.1016/j.jnoncrysol.2006.05.042
Morales-Acosta MD, Alvarado-Beltrán CG, Quevedo-López MA, Gnade BE, Mendoza-Galván A, Ramírez-Bon R (2013) Adjustable structural, optical and dielectric characteristics in sol-gel PMMA-SiO2 hybrid films. J Non Cryst Solids 362(1):124–135. https://doi.org/10.1016/j.jnoncrysol.2012.11.025
Melgoza-Ramírez ML, Ramírez-Bon R (2021) Europium ions as a spectroscopic probe in the study of PMMA-SiO2 hybrid microstructure with variable coupling agent. J Sol Gel Sci Technol, https://doi.org/10.1007/s10971-021-05582-2
El-bashir SM (2012) Photophysical properties of fluorescent PMMA/SiO2 nanohybrids for solar energy applications. J Lumin 132(7):1786–1791. https://doi.org/10.1016/j.jlumin.2012.02.010
Muthurasu A, Ganesh V (2021) Tuning optical properties of nitrogen-doped carbon dots through fluorescence resonance energy transfer using Rhodamine B for the ratiometric sensing of mercury ions. Anal Methods 13(15):1857–1865. https://doi.org/10.1039/d1ay00068c
Fang Q et al. (2017) Luminescence origin of carbon based dots obtained from citric acid and amino group-containing molecules. Carbon N Y 118:319–326. https://doi.org/10.1016/j.carbon.2017.03.061
Carbonaro CM et al. (2018) Carbon dots in water and mesoporous matrix: chasing the origin of their photoluminescence. J Phys Chem C 122(44):25638–25650. https://doi.org/10.1021/acs.jpcc.8b08012
Zhang X et al. (2013) Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS Nano 7(12):11234–11241. https://doi.org/10.1021/nn405017q
Wang H et al. (2016) Nitrogen-doped carbon dots for ‘green’ quantum dot solar cells. Nanoscale Res Lett 11(1):1–6. https://doi.org/10.1186/s11671-016-1231-1
Im HG, Park GU, Park HY, Jin J, Kang DJ (2017) A robust transparent protective hard-coating material using physicochemically-incorporated silica nanoparticles and organosiloxanes. Prog Org Coat 105:330–335. https://doi.org/10.1016/j.porgcoat.2017.02.004
Liu H et al. (2018) Scattering enhanced quantum dots based luminescent solar concentrators by silica microparticles. Sol Energy Mater Sol Cells 179:380–385. https://doi.org/10.1016/j.solmat.2018.01.029
Kadhim MA, Al-bermany E (2021) New fabricated PMMA-PVA/graphene oxide nanocomposites: structure, optical properties and application. J Composite Mater https://doi.org/10.1177/0021998321995912
Skuja L (1998) Section 1. Defect studies in vitreous silica and related materials: optically active oxygen-deficiency-related centers in amorphous silicon dioxide. J Non Cryst Solids 239(1–3):16–48. https://doi.org/10.1016/s0022-3093(98)00720-0
Fournier J et al. (2010) Evidence of a green luminescence band related to surface flaws in high purity silica glass. Opt Express 18(21):21557. https://doi.org/10.1364/oe.18.021557
Kang KS, Kim JH (2008) Origin of blue luminescence from a hybrid sol-gel after thermal processing. J Phys Chem C 112(2):618–620. https://doi.org/10.1021/jp068970h
Uchino T, Kurumoto N, Sagawa N (2006) Structure and formation mechanism of blue-light-emitting centers in silicon and silica-based nanostructured materials. Phys Rev B 73(23):1–4. https://doi.org/10.1103/PhysRevB.73.233203
García JM, Mondragón MA, Téllez CS, Camper A, Castaño VM (1995) Blue emission in tetraethoxysilane and silica gels. Mater Chem Phys 41(95):15–17
Brites CDS, Freitas VT, Ferreira RAS, Millán A, Palacio F, Carlos LD (2012) Metal-free highly luminescent silica nanoparticles. Langmuir 28(21):8190–8196. https://doi.org/10.1021/la300288j
Carlos LD, Sá Ferreira RA, Pereira RN, Assunção M, De Zea Bermudez V (2004) White-light emission of amine-functionalized organic/inorganic hybrids: emitting centers and recombination mechanisms. J Phys Chem B 108(39):14924–14932. https://doi.org/10.1021/jp049052r
Hinić I, Stanišić G, Popović Z (2003) Influence of the synthesis conditions on the photoluminescence of silica gels. J Serb Chem Soc 68(12):953–959. https://doi.org/10.2298/JSC0312953H
Glinka YD, Lin SH, Chen YT (2002) Time-resolved photoluminescence study of silica nanoparticles as compared to bulk type-III fused silica. Phys Rev B Condens Matter Mater Phys 66(3):354041–3540410. https://doi.org/10.1103/PhysRevB.66.035404
Molard Y et al. (2010) Red-NIR luminescent hybrid Poly(methyl methacrylate) containing covalently linked octahedral rhenium metallic clusters. Chem A Eur J 16(19):5613–5619. https://doi.org/10.1002/chem.200902131
Kuzema P, Bolbukh Y, Lipke A, Majdan M, Tertykh V (2019) Luminescent sol-gel glasses from silicate–citrate–(thio)ureate precursors. Colloids Interfaces 3(1):11. https://doi.org/10.3390/colloids3010011
Del Monte F, Levy D (1998) Formation of fluorescent rhodamine B J-dimers in sol-gel glasses induced by the adsorption geometry on the silica surface. J Phys Chem B 102(41):8036–8041. https://doi.org/10.1021/jp982396v
Chapman M, Euler WB (2018) Rhodamine 6G structural changes in water/ethanol mixed solvent. J Fluoresc 28:1431–1437
Arbeloa FL, Ojeda PR, Arbeloa IL (1988) On the aggregation of rhodamine B in ethanol. Chem Phys Lett 148(2):254–258
Liang Y et al. (2019) Hydrothermal growth of nitrogen-rich carbon dots as a precise multifunctional probe for both Fe3+ detection and cellular bio-imaging. Opt Mater 89:92–99. https://doi.org/10.1016/j.optmat.2019.01.008
Gutiérrez MC, Hortigüela MJ, Ferrer ML, Del Monte F (2007) Highly fluorescent rhodamine B nanoparticles entrapped in hybrid glasses. Langmuir 23(4):2175–2179. https://doi.org/10.1021/la0621057
Acknowledgements
The authors thank CONACyT for its support through the project Cátedra-CONACyT 1959. Itzel Arizbe Olivares-Torres gratefully acknowledges the Department of Physics, the Postgraduate in Nanotechnology of the University of Sonora, and CONACyT for the doctorate scholarship. We thank Marwan Abduljawad for his help with UV-Vis spectroscopy characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olivares-Torres, I.A., Alvarado-Rivera, J., Guzmán-Zamudio, R. et al. Adjustable emission of carbon dots and rhodamine B embedded in organic-inorganic hybrid gels for solid-state light devices. J Sol-Gel Sci Technol 106, 186–198 (2023). https://doi.org/10.1007/s10971-023-06048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06048-3